
Predictors and outcomes of ischemia-driven target lesion revascularization in deferred lesion based on fractional flow reserve: a multi-center retrospective cohort study
Introduction
Fractional flow reserve (FFR) has become the gold standard for the diagnosis of ischemia in angiographically intermediate epicardial coronary artery stenosis lesions and the use of percutaneous coronary intervention (PCI) (1,2). Although FFR, in addition to angiography, has been reported to be a valuable tool in improving long-term outcomes (3-5), adverse clinical events still occur in patients with high FFR (6). In fact, in the Fractional Flow Reserve Versus Angiography for Multivessel Evaluation (FAME) trial, the rate of major adverse cardiac events (MACE) in the FFR group was 13.2% at 1 year and 20% at 2 years (3,4).
Several papers have described ischemia-driven target lesion revascularization (TLR) in patients with deferral of revascularization based on FFR in the actual clinical setting (7-11).
On the other hand, several studies have also described that overall coronary atherosclerosis may also influence the incidence of MACE, irrespective of the FFR value, in deferral lesions (12,13). The synergy between percutaneous coronary intervention with taxus and cardiac surgery (SYNTAX) score is a lesion-based scoring system that predicts clinical outcomes after PCI in patients with multi-vessel coronary artery disease, on the basis of data derived from coronary angiograms alone (14,15). It is reportedly a reliable indicator of overall coronary atherosclerosis, and functional SYNTAX score has been thought to be a more effective index to accurately predict MACE (16). However, there is insufficient data on the impact of SYNTAX score on ischemia-driven TLR in deferral lesions. Therefore, in this retrospective cohort study, we investigated the predictors and clinical outcomes of deferral lesions in patients with angiographically intermediate epicardial coronary artery stenosis for which revascularization was postponed based on the FFR value. We present the following article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-773/rc).
Methods
Study population
In this retrospective cohort study, we assessed 440 patients (474 consecutive lesions) who underwent coronary angiography for acute coronary syndrome or stable angina pectoris and FFR for intermediate stenosis at nine centers between 2013 and 2017, and for whom data about the 3-year outcome were available.
All the hospitals have experienced and skilled doctors who have performed coronary artery angiopraphy on at least 1,000 cases per year. In all cases included in this study, revascularization was deferred based on FFR cut-off values of 0.80 or 0.75, as well as patient condition. The decision for deferral of revascularization was made by at least two experienced attending doctors specializing in coronary angiography.
However, we excluded patients with (I) cardiogenic shock, (II) chronic total occlusion lesion, (III) graft lesion, (IV) in-stent restenosis (ISR) with previous PCI history (≥2) (V) limited life expectancy due to comorbidity, (VI) drift more than 0.02, (VII) angiography by only single projection, or (VIII) severe valvular disease. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics committee of Tachikawa General Hospital (No. 19008). Data collection was approved by the local ethics committee, and written informed consent for analysis of anonymized data was obtained from all patients.
Coronary angiography
According to the respective local institutional guidelines, coronary angiography was performed using a 5-French (Fr) diagnostic or 6-Fr guiding catheter, and heparin was administered intravenously before the coronary angiography was performed. Quantitative coronary angiography was performed with optimal projections. The percentage diameter stenosis, minimum lumen diameter, reference vessel size, and lesion length were measured. In addition, lesions were classified into three types: focal, diffuse, and others. Angiographic focal lesions were defined according to previous reports (17-19). Calcified lesions were defined as fixed radiopaque densities seen in the area of the stenosis. These calcified lesions were graded as follows according to a previous report (20): mild, difficult to detect; moderate, easily identifiable; and heavy, when density was similar to that of the spine. According to coronary angiography, syntax score I was calculated based on previous reports (14,15).
FFR measurement
FFR was measured using a commercially available coronary pressure wire (Pressure Wire Certus, St Jude Medical, St Paul, AK; Prestige, Volcano Ltd, Cordova, CA). After administering intracoronary nitrates, the pressure wire was advanced into the site distal to the stenosis. According to the respective local institutional guidelines, maximal hyperemia was induced through intravenous infusion of adenosine triphosphate (ATP) (150–180 µg/kg body weight per minute) via the forearm or femoral vein, or through intracoronary injection of either ATP (40–80 µg), papaverine (8–12 mg), or nicorandil (2 mg). The drug was used at the discretion of the attending cardiologist. FFR was calculated as the ratio of the mean distal coronary pressure to the mean aortic pressure during maximum hyperemia.
Definitions
Individual patient data on clinical outcomes at 3 years were collected and analyzed. The primary outcome was ischemia-driven TLR in deferral lesion within 3 years. In addition, clinical outcomes, including cardiovascular death and myocardial infarction, which occurred in any coronary arteries within 3 years, were also assessed. Death was regarded as cardiac death unless other non-cardiac causes could be identified. Myocardial infarction was defined according to new or presumed new significant ST-segment-T wave changes, left bundle branch block, pathological Q waves in the electrocardiogram (ECG), imaging evidence of new viable myocardium loss, or any new regional wall motion abnormality identified as an intracoronary thrombus by angiography and an elevation of high-sensitive troponin T level.
ISR was defined as diameter stenosis ≥50% in the vessel segment within the stent or within 5 mm proximal or distal to the stent (21).
In the present study, analysis of death and myocardial infarction outcomes was performed on a patient level. In contrast, analysis of ischemia-driven TLR was performed on lesion level.
Statistical analysis
All statistical analyses were performed using IBM SPSS version 22 (IBM Japan Corp, Tokyo, Japan). Data are presented as number (percent) or mean ± SD.
The patients were divided into two groups based on a median FFR value of 0.86. Overall, the event-free and survival-free curves from clinical outcomes, including ischemia-driven TLR after FFR measurement and all-cause death, were estimated using the Kaplan-Meier method. Thereafter, the event-free curves from clinical outcomes for ischemia-driven TLR after FFR measurement were evaluated in subsets, such as acute coronary syndrome and stable angina pectoris.
In addition, a receiver operating characteristic (ROC) curve analysis was used to identify the SYNTAX score cut-off value for predicting an ischemia-driven TLR in deferral lesions. Furthermore, univariate cox proportional hazard model was performed. Thereafter, using covariates of P<0.05, multivariate cox proportional hazard model was conducted to identify predictors for ischemia-driven TLR. Finally, a sensitivity analysis for each gender was used to assess the validity of the study results. For all analyses, a two-sided P value of <0.05 was considered statistically significant.
Results
Baseline characteristics
Table 1 summarizes the baseline characteristics of the 440 consecutive patients, while Table 2 summarizes the location characteristics of the lesions and the FFR measurements of the 474 lesions analyzed in this study. The mean follow-up term was 973±213 days.
Table 1
| Variables | Number of patients (N=440) |
|---|---|
| Age (years) (mean ± SD) | 69.7±10.4 |
| Sex (male), n (%) | 306 (69.5) |
| BMI (kg/m2) (mean ± SD) | 25.1±4.8 |
| Hypertension, n (%) | 338 (76.8) |
| Diabetes, n (%) | 159 (36.1) |
| Dyslipidemia, n (%) | 283 (64.3) |
| Smoking history, n (%) | 248 (56.3) |
| Prior PCI, n (%) | 146 (33.1) |
| Prior CABG, n (%) | 8 (1.8) |
| Family history of ischemic heart disease, n (%) | 61 (13.9) |
| Chronic kidney disease, n (%) | 157 (35.7) |
| Hemodialysis, n (%) | 25 (5.7) |
| Clinical presentation, n (%) | |
| Stable angina | 298 (67.7) |
| Acute coronary syndrome | 76 (17.3) |
| STEMI | 4 (0.9) |
| NSTEMI | 32 (7.3) |
| Unstable angina | 40 (9.1) |
| Unknown | 66 (15.0) |
SD, standard deviation; BMI, body mass index; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; ACS, acute coronary syndrome; STEMI, ST-elevation myocardial infarction; NSTEMI, non-ST-elevation myocardial infarction.
Table 2
| Variables | Number of lesions (N=474) |
|---|---|
| Location of target lesions, n (%) | |
| Left main coronary artery | 18 (3.8) |
| Left anterior descending coronary artery | 273 (57.6) |
| Left circumflex coronary artery | 86 (18.1) |
| Right coronary artery | 95 (20.0) |
| ACC/AHA lesion classification, n (%) | |
| A | 226 (47.7) |
| B1 | 98 (20.7) |
| B2 | 66 (13.9) |
| C | 84 (17.7) |
| Vessel morphology, n (%) | |
| Focal (≤10 mm) | 307 (64.8) |
| Diffuse (>20 mm) | 66 (13.9) |
| Other | 101 (21.3) |
| Calcification score, n (%) | |
| None | 77 (16.2) |
| Mild | 307 (64.8) |
| Moderate | 66 (13.9) |
| Severe | 24 (5.1) |
| In-stent restenosis, n (%) | 28 (5.9) |
| SYNTAX score, mean ± SD | 7.2±4.2 |
| Mean FFR, mean ± SD | 0.8±0.05 |
| Median FFR [IQR] | 0.86 [0.83–0.89] |
| FFR categories, n (%) | |
| ≤0.8 | 89 (18.8) |
| 0.81–0.85 | 161 (34.0) |
| 0.86–0.90 | 184 (38.8) |
| 0.91–1.0 | 40 (8.4) |
| Quantitative coronary analysis result, mean ± SD | |
| Minimal lumen diameter (mm) | 1.5±0.4 |
| Lesion length (mm) | 14.0±8.4 |
| Diameter stenosis (%) | 50.2±12.7 |
SD, standard deviation; ACC/AHA, American College of Cardiology/American Heart Association; FFR, fractional flow reserve.
The average patient age was 69.7±10.4 years. Most of the patients were male (n=306, 69.5%). Left anterior descending coronary artery was the most prevalent culprit artery (273 cases, 57.6%). Relatively more simple lesions (Type A) were included. The average SYNTAX score was 7.2±4.2. There were 28 cases of ISR after drug-eluting stent (DES) implantation (5.9%). The prevalence of ISR was not associated with the amount of prior PCI history. Approximately 70% of patients had stable angina pectoris. Overall, the mean FFR value and median FFR [IQR] were 0.86±0.05 and 0.86 [0.83–0.89], respectively.
Clinical data and predictors for TLR in deferred lesions based on FFR value
As shown in Table 3, from a lesion perspective, the 3-year ischemia-driven TLR was 18 lesions (3.8%), which consisted of 18 patients. The median time of TLR was 381 [155–751]. However, on a patient basis, myocardial infarction occurred in 9 patients (2.0%), and there were 11 cases of all-cause death, of which cancer and aspiration pneumonia were the main causes (data not shown). On the contrary, cardiovascular death rate was very low (1 case). Five out of 28 cases of ISR (17.8%) developed the 3-year ischemia-driven TLR.
Table 3
| Major adverse cardiac events | Number |
|---|---|
| Patient level, n (%) | N=440 |
| All-cause mortality | 11 (2.5) |
| Cardiovascular death | 1(0.2) |
| Stroke | 7(1.6) |
| Myocardial infarction | 9 (2.0) |
| Lesion level, n (%) | N=474 |
| Ischemia driven-TLR | 18 (3.8) |
TLR, target lesion revascularization.
Overall, Kaplan-Meier curves for freedom from all-cause death, myocardial infarction (MI), and ischemia-driven TLR during the 3-year follow-up were analyzed according to a median FFR value of 0.86. For ischemia-driven TLR, the deferral group with a low FFR value tended to have higher ischemia-driven TLR than that with a high FFR value (Log-Rank P=0.09) (Figure 1).

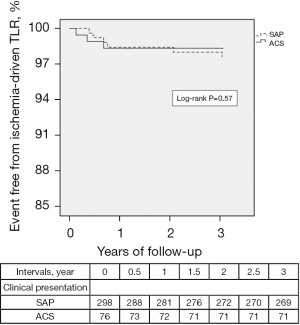
In addition, though Kaplan-Meier curves for freedom from ischemia-driven TLR were compared in subsets, such as acute coronary syndrome and stable angina pectoris, there was no difference between the two groups (Figure 2).
In univariate cox proportional hazard model, syntax score, ISR, minimum lumen diameter, FFR value, and left main coronary artery lesion were identified as covariates of P<0.10, as shown in Table 4.
Table 4
| Variables | Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age | 0.99 | 0.95–1.03 | 0.80 | ||||
| Sex (female) | 1.54 | 0.50–4.69 | 0.44 | ||||
| BMI | 1.00 | 0.90–1.10 | 0.96 | ||||
| Family history of ischemic heart disease | 0.36 | 0.05–2.71 | 0.32 | ||||
| Smoker | 1.59 | 0.59–4.24 | 0.35 | ||||
| Hypertension | 0.60 | 0.22–1.62 | 0.31 | ||||
| Diabetes | 1.13 | 0.44–2.93 | 0.79 | ||||
| Dyslipidemia | 0.84 | 0.32–2.18 | 0.73 | ||||
| Acute coronary syndrome | 0.45 | 0.12–1.57 | 0.21 | ||||
| Hemodialysis | 2.36 | 0.54–10.29 | 0.25 | ||||
| SYNTAX score | 1.08 | 1.01–1.17 | 0.04 | 1.10 | 1.01–1.19 | 0.03 | |
| Left main | 1.80 | 0.86–3.76 | 0.09 | ||||
| LAD | 0.98 | 0.72–1.36 | 0.94 | ||||
| LCX | 0.89 | 0.62–1.29 | 0.56 | ||||
| RCA | 0.89 | 0.26–3.09 | 0.86 | ||||
| FFR value (median value >0.86) | 0.40 | 0.11–1.18 | 0.09 | ||||
| Focal lesion >20 mm | 0.74 | 0.26–2.08 | 0.57 | ||||
| Moderate to severe calcified lesion | 0.66 | 0.13–3.30 | 0.61 | ||||
| In-stent restenosis | 6.10 | 2.17–17.13 | <0.01 | 6.33 | 2.25–17.8 | <0.01 | |
| Minimal lumen diameter | 0.30 | 0.08–1.18 | 0.08 | ||||
| Calcium score ≥2 | 0.66 | 0.19–2.28 | 0.52 | ||||
TLR, target lesion revascularization; HR, hazard ratio; CI, confidence interval; BMI, body mass index; ACC/AHA, American College of Cardiology/American Heart Association; FFR, fractional flow reserve; LAD, left anterior descending coronary artery; LCX, left circumflex artery; RCA, right coronary artery.
Finally, according to multivariate cox proportional hazard model, SYNTAX score and ISR were independent predictors for TLR in deferral lesions [hazard ratio (HR)=1.10, 95% confidential interval (CI): 1.01–1.19, P=0.03; HR =6.33; 95% CI: 2.25–17.8, P<0.01, respectively] (Table 4). Likewise, sensitivity analysis for each gender was used to assess the validity of the study result, which resulted in similar findings (data not provided).
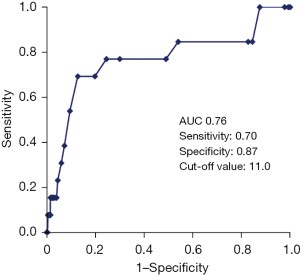
ROC curve analysis showed that the cut-off value of SYNTAX score to predict ischemia-driven TLR in deferral lesions was 11.0 (Figure 3).
Discussion
The study shows that SYNTAX score and ISR were associated with TLR in deferral lesions at 3 years. Several reports on the clinical outcomes of patients with revascularization deferral based on FFR in the clinical setting exist; however, the present study is the first to report an association between SYNTAX score and ischemia-driven TLR in deferral lesions.
The principle of physiological guidance of revascularization is to identify lesions in which deferral is likely to be safe (3). Moreover, PCI for coronary stenosis without inducible ischemia (FFR >0.80) may not improve the prognosis (22).
According to recent randomized and observational studies, FFR has been shown to successfully identify lesions that can be safely managed conservatively among angiographically moderate lesions (4,5,9,23,24). The usefulness of FFR has been emphasized in the above-mentioned studies, including those conducted in Japan (7,8).
In the present study, the prevalence of TLR of deferral lesion was approximately 4%, which was comparable to that of previously published trials from Japan (7,8).
In previous reports, from lesion-specific and patient perspectives, lower FFR value, moderately to severely calcified lesion, minimum lumen diameter, diabetes mellitus, hemodialysis, left main coronary artery lesion, right coronary artery lesion, and acute coronary syndrome have been reported as predictors for TLR of deferral lesion based on FFR (7,8,10,11). In the present study, as shown in Figure 1, a lower FFR value was associated with a higher incidence of ischemia-driven TLR in deferral lesions by Kaplan Meyer analysis, which was based on median FFR value, in line with the previous report (7). Although the left main coronary artery and minimum lumen diameter tended to have higher ischemia-driven TLR in the univariate analysis, only minimum lumen diameter tended to be a predictor for ischemia-driven TLR, not left main coronary artery, hemodialysis, or diabetes mellitus in the present study. However, hemodialysis and diabetes mellitus are reportedly associated with coronary events (25-27). Though speculative, these factors did not remain significant probably because the duration of hemodialysis was not considered, and the definition of diabetes mellitus without considering kinds of medications and diabetes control level might have influenced these results. In addition, since only 18 left main coronary artery cases were enrolled, the statistic power may be insufficient to elucidate whether the left main coronary artery could predict TLR. Furthermore, regarding the acute coronary syndrome clinical setting, the reliability of FFR values to the non-culprit artery at an acute phase in a case of ST elevated myocardial infarction remains controversial (28-31). In the present study, though the incidence of ischemia-driven TLR was compared by the breakdown of subsets such as acute coronary syndrome and stable angina pectoris, there was no difference between the two groups. However, ST-elevation myocardial infarction in the acute coronary syndrome group consisted of only 4 cases. Therefore, a definite conclusion was not drawn from the present findings. In addition, the benefit of using FFR to guide PCI in multi-vessel disease in unstable angina and non-ST-elevation myocardial infarction may be different from that in ST-elevation myocardial infarction.
On the other hand, in the present study, SYNTAX score and ISR were identified as novel predictors for TLR after deferral of revascularization based on FFR. SYNTAX score has been reported to predict clinical outcomes after PCI in patients with multi-vessel coronary artery disease (14,15). Furthermore, a previous report has described that the low-FFR (<0.80) group had more severe stenosis and higher SYNTAX scores (median value 14.0) (6). In addition, functional SYNTAX scores have also been effective in predicting better prognosis in patients with multi-vessel coronary artery disease undergoing PCI (16). Thus, these reports may indicate it is crucial to incorporate the anatomical complexity of the coronary artery into a functional evaluation of such, which suggests the present findings are aligned with the reports mentioned above.
In addition, in the 3V (three-vessel) FFR-FRIENDS trial, the low 3V-FFR group showed a higher event rate than the high 3V-FFR group. The low 3 V-FFR was also an independent predictor of MACE (13). Thus, considering these findings and those of the present study, special attention should be given not only to the focal stenotic lesion but also to the overall atherosclerotic conditions, using FFR and SYNTAX score.
In terms of ISR, previous reports have described that FFR measurement in patients with restenosis after bare metal stent implantation and DES seems to be useful in treatment decision making (32,33). Though there is insufficient data describing ISR as an independent predictor of TLR after the deferral based on FFR value, a study reporting the 12-month clinical outcomes of ISR lesions according to FFR showed that deferral lesions (FFR ≥0.80) demonstrated tendency toward lower incidence of MACE in ISR lesions after DES implantation (32). However, the incidence rate was 10% even in an ISR lesion with FFR ≥0.80. This showed a higher incidence rate than that of the TLR rate of approximately 5% in deferred native lesions (7,8,10). In fact, various factors affecting the outcome of ISR have been reported, which includes under-expansion and neointimal hyperplasia including neoatherosclerosis (34-37). Therefore, even in ISR cases with a higher FFR value, the rate of ischemia-driven TLR may be high compared to native lesions, as shown in this study.
Taken together, in the present study, the incidence of TLR in deferral lesions was low at 3.8%, which supports the efficacy of FFR in clinical practice. However, the study indicates that TLR events are more likely to occur in lesions with lower FFR value. In addition, SYNTAX score and ISR were identified as independent predictors for TLR. Therefore, these factors should be considered in patient treatment. In addition, optimal medical therapy should be performed, especially for those with high risk factors.
Limitations
The present study had a relatively small number of patients compared to that of previous studies. Second, the present study included a wide selection of clinical presentation, including ST-elevation myocardial infarction and stable angina pectoris. It is known that the incidence rate of cardiovascular events can vary based on clinical presentations (10,38), which may have affected our results. Third, coronary plaque has been reported to affect coronary events (39-41). Therefore, in the present study, intracoronary imaging data should have been collected. Fourth, optimal medical therapy is essential to prevent future cardiac events in patients with deferral lesions; however, it could not be determined whether the medical therapy during follow-up was optimal. Fifth, the present study included only deferral patients according to FFR results, which may have introduced selection bias because they differed according to local institutional guidelines. Therefore, the reasons for the deferral should have been clarified. However, in one of the largest studies performed in Japan, the CVIT-DEFER Registry (8), 506 out of 3,857 lesions were enrolled as FFR <0.80, consistent with the present study results. Furthermore, since this is a high-volume multi-center study, a detailed flow diagram for the entry of patients should have been made. Because the present study is a real-world retrospective study, we must acknowledge this as a limitation. However, in the present study, SYNTAX score and ISR were independent predictors for ischemia-driven TLR of deferral patients. Thus, these findings may be useful in the management of such patients.
Finally, further prospective studies involving more patients to investigate the threshold of SYNTAX score and FFR value are warranted.
Conclusions
Lesions with lower FFR were associated with higher incidence of ischemia-driven TLR than those with higher FFR. Moreover, SYNTAX score and ISR were associated with ischemia-driven TLR at 3 years.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-773/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-773/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-773/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-773/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics committee of Tachikawa General Hospital (approval ID 19008). Data collection was approved by the local ethics committee, and written informed consent for analysis of anonymized data was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pijls NH, De Bruyne B, Peels K, et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med 1996;334:1703-8. [Crossref] [PubMed]
- Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87-165. [Crossref] [PubMed]
- Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 2009;360:213-24. [Crossref] [PubMed]
- Pijls NH, Fearon WF, Tonino PA, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study. J Am Coll Cardiol 2010;56:177-84. [Crossref] [PubMed]
- Zimmermann FM, Ferrara A, Johnson NP, et al. Deferral vs. performance of percutaneous coronary intervention of functionally non-significant coronary stenosis: 15-year follow-up of the DEFER trial. Eur Heart J 2015;36:3182-8. [Crossref] [PubMed]
- Lee JM, Jung JH, Hwang D, et al. Coronary Flow Reserve and Microcirculatory Resistance in Patients With Intermediate Coronary Stenosis. J Am Coll Cardiol 2016;67:1158-69. [Crossref] [PubMed]
- Kuramitsu S, Matsuo H, Shinozaki T, et al. Two-Year Outcomes After Deferral of Revascularization Based on Fractional Flow Reserve: The J-CONFIRM Registry. Circ Cardiovasc Interv 2020;13:e008355. [Crossref] [PubMed]
- Tanaka N, Nakamura M, Akasaka T, et al. One-Year Outcome of Fractional Flow Reserve-Based Coronary Intervention in Japanese Daily Practice - CVIT-DEFER Registry. Circ J 2017;81:1301-6. [Crossref] [PubMed]
- Weerts J, Pustjens T, Amin E, et al. Long-term outcome after deferred revascularization due to negative fractional flow reserve in intermediate coronary lesions. Catheter Cardiovasc Interv 2021;97:247-56. [Crossref] [PubMed]
- Escaned J, Ryan N, Mejía-Rentería H, et al. Safety of the Deferral of Coronary Revascularization on the Basis of Instantaneous Wave-Free Ratio and Fractional Flow Reserve Measurements in Stable Coronary Artery Disease and Acute Coronary Syndromes. JACC Cardiovasc Interv 2018;11:1437-49. [Crossref] [PubMed]
- Hoshino M, Hamaya R, Kanaji Y, et al. Sex Differences in Long-Term Outcomes in Patients With Deferred Revascularization Following Fractional Flow Reserve Assessment: International Collaboration Registry of Comprehensive Physiologic Evaluation. J Am Heart Assoc 2020;9:e014458. [Crossref] [PubMed]
- Hokama Y, Tanaka N, Takashima H, et al. Insufficient recovery of fractional flow reserve even after optimal implantation of drug-eluting stents: 3-year outcomes from the FUJI study. J Cardiol 2021;77:532-8. [Crossref] [PubMed]
- Lee JM, Koo BK, Shin ES, et al. Clinical implications of three-vessel fractional flow reserve measurement in patients with coronary artery disease. Eur Heart J 2018;39:945-51. [Crossref] [PubMed]
- Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009;360:961-72. [Crossref] [PubMed]
- Ong AT, Serruys PW, Mohr FW, et al. The SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery (SYNTAX) study: design, rationale, and run-in phase. Am Heart J 2006;151:1194-204. [Crossref] [PubMed]
- Nam CW, Mangiacapra F, Entjes R, et al. Functional SYNTAX score for risk assessment in multivessel coronary artery disease. J Am Coll Cardiol 2011;58:1211-8. [Crossref] [PubMed]
- Kini A, Marmur JD, Kini S, et al. Creatine kinase-MB elevation after coronary intervention correlates with diffuse atherosclerosis, and low-to-medium level elevation has a benign clinical course: implications for early discharge after coronary intervention. J Am Coll Cardiol 1999;34:663-71. [Crossref] [PubMed]
- Pijls NH, De Bruyne B, Bech GJ, et al. Coronary pressure measurement to assess the hemodynamic significance of serial stenoses within one coronary artery: validation in humans. Circulation 2000;102:2371-7. [Crossref] [PubMed]
- De Bruyne B, Pijls NH, Heyndrickx GR, et al. Pressure-derived fractional flow reserve to assess serial epicardial stenoses: theoretical basis and animal validation. Circulation 2000;101:1840-7. [Crossref] [PubMed]
- Ghazzal ZM, Hearn JA, Litvack F, et al. Morphological predictors of acute complications after percutaneous excimer laser coronary angioplasty. Results of a comprehensive angiographic analysis: importance of the eccentricity index. Circulation 1992;86:820-7. [Crossref] [PubMed]
- Gonzalo N, Serruys PW, Okamura T, et al. Optical coherence tomography patterns of stent restenosis. Am Heart J 2009;158:284-93. [Crossref] [PubMed]
- Pijls NH, van Schaardenburgh P, Manoharan G, et al. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol 2007;49:2105-11. [Crossref] [PubMed]
- Ahn JM, Park DW, Shin ES, et al. Fractional Flow Reserve and Cardiac Events in Coronary Artery Disease: Data From a Prospective IRIS-FFR Registry (Interventional Cardiology Research Incooperation Society Fractional Flow Reserve). Circulation 2017;135:2241-51. [Crossref] [PubMed]
- Fearon WF, Nishi T, De Bruyne B, et al. Clinical Outcomes and Cost-Effectiveness of Fractional Flow Reserve-Guided Percutaneous Coronary Intervention in Patients With Stable Coronary Artery Disease: Three-Year Follow-Up of the FAME 2 Trial (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation). Circulation 2018;137:480-7. [Crossref] [PubMed]
- Herzog CA, Ma JZ, Collins AJ. Poor long-term survival after acute myocardial infarction among patients on long-term dialysis. N Engl J Med 1998;339:799-805. [Crossref] [PubMed]
- Whiteley L, Padmanabhan S, Hole D, et al. Should diabetes be considered a coronary heart disease risk equivalent?: results from 25 years of follow-up in the Renfrew and Paisley survey. Diabetes Care 2005;28:1588-93. [Crossref] [PubMed]
- Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 2009;301:2129-40. [Crossref] [PubMed]
- Niccoli G, Indolfi C, Davies JE. Evaluation of intermediate coronary stenoses in acute coronary syndromes using pressure guidewire. Open Heart 2017;4:e000431. [Crossref] [PubMed]
- Smits PC, Abdel-Wahab M, Neumann FJ, et al. Fractional Flow Reserve-Guided Multivessel Angioplasty in Myocardial Infarction. N Engl J Med 2017;376:1234-44. [Crossref] [PubMed]
- Engstrøm T, Kelbæk H, Helqvist S, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3—PRIMULTI): an open-label, randomised controlled trial. Lancet 2015;386:665-71. [Crossref] [PubMed]
- Puymirat E, Cayla G, Simon T, et al. Multivessel PCI Guided by FFR or Angiography for Myocardial Infarction. N Engl J Med 2021;385:297-308. [Crossref] [PubMed]
- Nam CW, Rha SW, Koo BK, et al. Usefulness of coronary pressure measurement for functional evaluation of drug-eluting stent restenosis. Am J Cardiol 2011;107:1783-6. [Crossref] [PubMed]
- Lopez-Palop R, Pinar E, Lozano I, et al. Utility of the fractional flow reserve in the evaluation of angiographically moderate in-stent restenosis. Eur Heart J 2004;25:2040-7. [Crossref] [PubMed]
- de Feyter PJ, Kay P, Disco C, et al. Reference chart derived from post-stent-implantation intravascular ultrasound predictors of 6-month expected restenosis on quantitative coronary angiography. Circulation 1999;100:1777-83. [Crossref] [PubMed]
- Cheneau E, Leborgne L, Mintz GS, et al. Predictors of subacute stent thrombosis: results of a systematic intravascular ultrasound study. Circulation 2003;108:43-7. [Crossref] [PubMed]
- Kim JS, Lee JH, Shin DH, et al. Long-term outcomes of neointimal hyperplasia without neoatherosclerosis after drug-eluting stent implantation. JACC Cardiovasc Imaging 2014;7:788-95. [Crossref] [PubMed]
- Nakazawa G, Otsuka F, Nakano M, et al. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. J Am Coll Cardiol 2011;57:1314-22. [Crossref] [PubMed]
- Daida H, Miyauchi K, Ogawa H, et al. Management and two-year long-term clinical outcome of acute coronary syndrome in Japan: prevention of atherothrombotic incidents following ischemic coronary attack (PACIFIC) registry. Circ J 2013;77:934-43. [Crossref] [PubMed]
- Erlinge D, Maehara A, Ben-Yehuda O, et al. Identification of vulnerable plaques and patients by intracoronary near-infrared spectroscopy and ultrasound (PROSPECT II): a prospective natural history study. Lancet 2021;397:985-95. [Crossref] [PubMed]
- Prati F, Romagnoli E, Gatto L, et al. Relationship between coronary plaque morphology of the left anterior descending artery and 12 months clinical outcome: the CLIMA study. Eur Heart J 2020;41:383-91. [PubMed]
- Lee JM, Choi KH, Koo BK, et al. Prognostic Implications of Plaque Characteristics and Stenosis Severity in Patients With Coronary Artery Disease. J Am Coll Cardiol 2019;73:2413-24. [Crossref] [PubMed]


