Therapeutic hypothermia impacts leukocyte kinetics after cardiac arrest
Introduction
Cardiovascular diseases (CVDs) are the leading cause of death in developed countries. According to the World Health Organization (WHO), about 17.5 million people die from CVDs every year—80% due to stroke or myocardial infarction (MI) (1).
In very severe cases these entities can lead to cardiac arrest (CA) with need of cardiopulmonary resuscitation (CPR). Even if return of spontaneous circulation (ROSC) is achieved, a high mortality remains in these patients (2). Therefore, optimized post-resuscitation care including targeted temperature management (TTM) for mild hypothermia is crucial for survival as well as neurological outcome (3).
Initially, TTM was recommended in patients who were admitted in a comatose status after successful CPR especially with shockable rhythms (VF, pVT) in initial electrocardiography (ECG) registration (4,5). In recent years, evidence emerged that this intervention could also be beneficial in patients with initial non-shockable rhythms. Perman and colleagues have lately shown that a benefit in survival and neurologic outcome at hospital discharge is observed in this group of patients (6).
The most common cause of CA in adult patients is ischemic CVD (2,7). The immune response following acute MI plays an important role in the quality of healing (8,9). An elevated leukocyte count in the acute phase is associated with complications as well as short- and long-term mortality (10,11). In this context leukocytes can act in a beneficial as well as a harmful manner, depending on subclasses, timing and circumstances (12,13). Therefore, influencing leukocyte mediated inflammation seems to be a potential target for therapeutic approaches (14).
It has been shown that therapeutic hypothermia suppresses leukocyte counts in encephalopathic neonates (15). Additionally, several in vitro and in vivo reports showed an impact of hypothermia on the immune system, especially on leukocyte function in a mainly inhibiting manner (16-20).
In the presented study, we investigated the effect of therapeutic hypothermia on leukocyte counts and C-reactive protein (CRP) levels and evaluated survival as well as neurological outcome after CA.
Methods
Study design
In this retrospective, single-center study we analyzed the course of leukocyte counts and CRP up to 7 days after CA in patients who underwent TTM for mild therapeutic hypothermia compared to those without active temperature management.
We included patients who were resuscitated due to non-traumatic reasons in a primarily successful manner and who were admitted to the intensive care unit of the University Hospital of Heidelberg. These patients were included into the “Heidelberg resuscitation-registry” (HRR), which is approved by the Heidelberg Medical Ethical Committee (S-388/2011) and complies with the declaration of Helsinki. Informed consent was obtained from the legal guardians or immediate family. Medical treatment was performed following standard operating procedures based on current guidelines and literature.
When indicated, TTM for therapeutic hypothermia was carried out with an endovascular cooling device (Coolgard 3000/ICY® catheter, Zoll Medical Corp, USA) and a target temperature of 32–34 °C was maintained. In one patient target temperature was adjusted to 36 °C following the decision of the treating physician. Targeted temperature was maintained for 24 h followed by a rewarming phase of 0.1–0.3 °C per hour until normothermia was reached.
Reasons to refrain from applying TTM included consciousness at time of presentation, short latency until CPR was administered, severe bleeding or bradycardia as well as unavailability of device.
In this analysis we included 169 patients which were admitted between May 2013 and April 2015 to the intensive care unit of our institution. Patients were followed up for at least 30 days either by a follow-up visit in our out-patient center or by telephone calls to patients themselves, their families, physicians or electronic hospital records. If possible, cerebral performance category (CPC) was additionally assessed 6 months after CPR. For statistical analyses a CPC score of 1 and 2 was considered to be a favorable neurological outcome whereas CPC 3 to 5 was classified as an unfavorable status (21).
In the first 30 days, 12 patients were lost to follow up in total, five of them out of the TTM group, and were therefore excluded from survival analyses. For CPC assessment after 6 months no follow up was possible in nineteen patients, nine being out of the TTM cohort.
The primary endpoint was set by death within 30 days after CA. As secondary endpoints the neurological outcome, the kinetics of leukocyte counts and CRP in cardiac patients as well as the predictive value of leukocyte counts undergoing TTM in terms of 30 days mortality and neurological outcome were specified.
Laboratory analyses
Laboratory analyses were done in the clinical core laboratory of the University Hospital of Heidelberg as part of the standardized daily routine in patients after CA. Blood was drawn on admission (d 0) and the early morning of the following days (d 1–7) in setting of a standardized routine.
Statistics
Chi Square test for categorical data and Mann-Whitney U test were performed for comparison of two groups. Mann-Whitney U test was applied as part of the data showed a non-parametric distribution in the D’Agostino & Pearson test.
To determine cut-off levels, Youden’s J statistic was calculated out of receiver operating characteristic (ROC) curves.
For assessment of risk factors a logistic regression model was applied (stepwise). Additionally survivorship was analyzed using Cox proportional hazard regression. Evaluated risk factors for death and neurological outcome included age, sex and TTM if not otherwise stated. Age was box-cox transformed as it showed a skewed distribution.
A P value<0.05 was considered statistically significant. Analyses were performed with GraphPad Prism V6 (GraphPad Software, Inc., USA) and MedCalc Statistical Software Version 16.1 (MedCalc Software bvba, Ostend, Belgium).
Results
Study cohort
In this retrospective study we analyzed data of 169 patients following CA due to non-traumatic reasons, who were admitted to the intensive care unit of the University Hospital of Heidelberg between May 2013 and April 2015 (Table 1). Of this cohort 111 subjects underwent TTM for mild therapeutic hypothermia. The mean age was lower in TTM treated patients compared to those without TTM (70.2 vs. 59.8 years; P<0.0001). Male patients accounted for 69.8% of the whole population and 77.5% in the TTM group (P=0.0028).
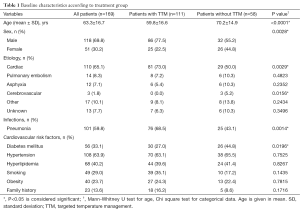
Full table
The etiology of CA was predominantly of cardiac origin, including particularly MI and cardiomyopathies as well as myocarditis and primary ventricular arrhythmias. Those cardiac causes were responsible for up to 73% of CA in the TTM group, which was significantly more than in the non-hypothermia cohort (P=0,029). Except for intracerebral haemorrhage, when hypothermia is relatively contraindicated, there was no statistically significant difference in the other etiologies concerning TTM.
Regarding cardiovascular risk factors, 63.9% of the patients suffered from arterial hypertension while in 40.2% hyperlipidemia and in 33.1% diabetes mellitus was reported.
A meta-analysis by Geurts and colleagues demonstrated an increased risk for pneumonia in patients treated with hypothermia (22). This finding was corroborated in our study group: The occurrence of pneumonia was significantly higher in patients receiving TTM (68.5%) compared to non-hypothermic patients (43.1%; P=0.0014) with a relative risk of 1.58 [95% confidence interval (CI), 1.160–1.941].
Therapeutic hypothermia improves survival and neurological outcome after CA
As recommend by current guidelines (23,24), all patients who were admitted to our hospital following non-traumatic CA were evaluated concerning TTM for mild hypothermia, independent of the first registered heart rhythm. To evaluate if TTM has a benefit on survival independent of other factors like age and sex we performed Cox proportional hazard regression analyses. As age showed a skewed distribution it was box-cox transformed beforehand. Figure 1A illustrates the outcome dependent on TTM within the first 30 days after CA for all patients. A significant difference in survival of patients undergoing TTM is observed in the acute phase and the survival gap widens over time [P=0.0001; hazard ratio (HR), 2.2479; 95% CI, 1.4983–3.3724]. This beneficial effect of TTM was independent from other factors like age and sex. The analysis further revealed that in contrast to sex (P>0.05), age is another independent factor for survival (P=0.0050; HR, 1.0008; 95% CI, 1.0003–1.0014). In addition, the survival curve stresses the importance of the first 15 days after CA for longer-term survival.

As described above, a higher incidence of pneumonia was observed in our TTM cohort compared to patients who did not receive cooling therapy. To further assess if this common complication has an effect on survival we established a logistic regression model including TTM, age, sex and presence of pneumonia. Again TTM and age showed to be independent predictors for survival [TTM: P=0.0014; odds ratio (OR), 4.7327; 95% CI, 1.8181–12.3198; age: P=0.0030; OR, 1.0017; 95% CI, 1.0006–1.0028; area under the curve (AUC), 0.748] while pneumonia and sex were not (both P>0.05).
Since hypoxic brain injury following CA is a major predicament and TTM is considered to ameliorate neurological outcome (3) we conducted further analyses to evaluate the effect of TTM on CPC in our patients. CPC-categories 1 and 2 at 6 months post CA were considered as beneficial while CPC-categories 3 to 5 as unfavorable neurological outcome. Logistic regression analyses revealed that withholding TTM and older age are independent predictors for inferior neurological performance (TTM: P=0.0030; OR, 0.1495; 95% CI, 0.0426–0.5244; age: P=0.0084; OR, 0.9984; 95% CI, 0.9972–0.9996; sex: P>0.05; AUC, 0.756). Expansion of this regression model by pneumonia (P>0.05) resulted in no significant changes, indicating that the presence of this infective complication has no predictive value for poor neurological outcome.
As described above cardiac pathogenesis accounts for the main reason of CA in adult patients. Therefore we further assessed this cohort of patients separately and excluded those with non-cardiac causes of CA from further analysis (Figure 1B). In this cohort again TTM was an independent variable for a significant better survival in the first 30 days after CA compared to the non-hypothermia group (P=0.0145; HR, 2.0068; 95% CI, 1.1483–3.5070). Again, age was shown to be a significant factor (P=0.0002; HR, 1.0016; 95% CI, 1.0007–1.0024) whereas sex failed to do so (P>0.05). Logistic regression additionally including pneumonia showed similar results to the ones in patients with all etiologies (TTM: P=0.0307; OR, 3.8245; 95% CI, 1.1331–12.9086; age: P=0.0005; OR, 1.0028; 95% CI, 1.0012–1.0044; sex and pneumonia: P>0.05; AUC, 0.777).
Cooling after CA affects leukocyte and CRP kinetics differently
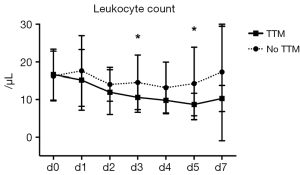
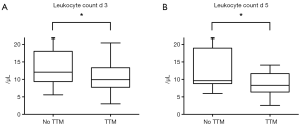
As inflammation plays a critical role in various cardiovascular conditions and leukocytes predict outcome in acute cardiovascular events (10,11) we further assessed the course of leukocytes in patients of the cardiac subgroup undergoing hypothermia following CPR. Having suffered from CA due to a cardiac event, mean leukocyte counts on admission were 16.5±6.7 per microliter in all of these patients combined. Analysis of both cohorts showed no significant differences at baseline (no TTM group: 16.3 vs. 16.6 µL in TTM treated patients; P=0.9224). While leukocytes remained fairly stable in patients following CA without TTM (leukocyte count on day 5 in normothermic patients: 14.3±9.7 µL), we observed a significant reduction in patients who received cooling therapy (Figure 2). In those patients with maintained hypothermia, leukocyte levels declined gradually over the first 5 days after CPR, reaching its minimum on day 5 after CA. Interestingly, differences in leukocyte counts started to be apparent during hypothermia but also remained significant beyond the time period of TTM. Analysis for day 3 (14.6±7.3 vs. 10.6±4.0 µL, P=0.0400) and day 5 (14.3±9.7 vs. 8.7±3.0 µL, P=0.0159) after CA is shown in Figure 3.
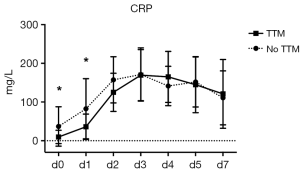
To further validate the effect of TTM on inflammation, we performed serial measurements of CRP and found a significant difference in both cohorts at baseline (d 0, 36.9±50.9 vs. 9.8±17.3 mg/L, P=0.0001) and on day 1 after CPR (82.7±77.7 vs. 36.2±32.7 mg/L, P=0.0388). In contrary to the above described influence on leukocyte kinetics however, no significant differences in CRP levels were detected between the two groups once the rewarming phase is initiated (Figure 4; d 2 P>0.1013, from d 3 on P>0.2).
Leukocyte count as a prognostic marker in patients undergoing TTM after CA
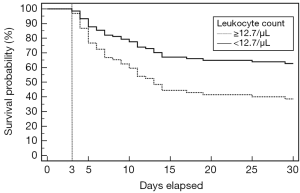
Based on these findings, we performed a ROC analysis for leukocyte levels on day 3 in all patients undergoing TTM after CA and assessed a cut-off value by Youden’s J statistic of 12.7/µL with a sensitivity of 76% and specificity of 51%. A Cox regression model including age and sex showed that a leukocyte count below this cut-off level on day three post CA in patients undergoing TTM is an independent predictor for survival for up to 30 days (Figure 5; for TTM: P=0.0214; HR, 2.0385; 95% CI, 1.1115–3.7388; for age: P=0.0296; HR, 1.0012; 95% CI, 1.0001–1.0022; no significance for sex as P>0.05). Furthermore, applying this threshold results in a predictive distinction between CPC 1–2 and CPC 3–5 6 months after resuscitation and therefore might serve as a prognostic value for neurological outcome as well (P=0.0439; OR, 0.3676; 95% CI, 0.1389–0.9732; age: P=0.0249; OR, 0.9983; 95% CI, 0.9968–0.9998; sex: P>0.05; AUC, 0.698). In contrast, pneumonia was not found to be an independent predictor when added to the corresponding logistic regression models for survival (P>0.05 for pneumonia and sex; P=0.0084; OR, 3.6049; 95% CIM 1.3882–9.3611 for leukocyte cut-off; P=0.0103; OR, 1.0020; 95% CI, 1.0005–1.0036 for age; AUC, 0.726) and neurological outcome (P>0.05 for pneumonia and sex).
Discussion
The treatment of patients who survive CA and CPR remains a difficult challenge in clinical practice. While the implementation of new treatment approaches of acute MI—the most common cause for CA—have resulted in a markedly improved survival in recent decades (25), the survival rate of out-of-hospital CA remains at a devastating low of 9.5% (26). Therapeutic hypothermia has been shown to improve neurological outcome and survival after CA, regardless of the initial heart rhythm detected and is recommended in international guidelines (23,24). In our analysis of 169 patients that were admitted to the intensive care unit of the University Hospital of Heidelberg due to non-traumatic CA and following resuscitation we found that therapeutic hypothermia with a target temperature of 32–34 °C over the course of 24 hours significantly reduced mortality in the follow-up period of 30 days. Furthermore patients undergoing hypothermia showed a favorable long-term neurological outcome 6 months after CA.
One critical limitation of our study is differences in both groups of patients. In order to address this bias, we conducted logistic and Cox proportional hazard regression analyses that showed that the benefit of TTM is independent of other factors like age or sex.
It is yet undetermined if target temperature should be set at 36 °C rather than 32–34 °C, emphasizing the concept of avoiding fever instead of actively cooling down as the main goal. Recent data however showed no significant differences in terms of survival and neurological outcome between those two groups (27). Target temperatures between 32 °C and 36 °C in post-resuscitation care are therefore currently recommended (23,24).
An acute cardiac event is the most common cause for CA. Inflammation plays a critical role in the development of coronary heart disease and is centrally involved in healing after MI (28). While invading leukocytes are essential for the removal of debris and dead tissue, an excessive production of myeloid cells is associated with an impaired development of cardiac function and outcome (12,29,30). And as shown recently, an acute event like MI additionally accelerates established atherosclerosis due to a burst of acute systemic inflammation (31,32).
Several studies have investigated the effect of hypothermia on immune responses and leukocyte function in vitro and in vivo, some of them with conflicting results (16-20). In an experimental model of acute myocardial ischemia and subsequent CPR and hypothermia, cooling reduced myocardial damage and dysfunction via a reduced rate of apoptosis and pro-inflammatory cytokine expression (33). Elevated cytokine levels were reported in patients after CA under hypothermia (34,35), whereas the level of TTM seems to have no effect on systemic inflammatory cytokine responses (36).
We here report, for the first time to our knowledge, which therapeutic hypothermia leads to a reduction in leukocyte levels after CA due to cardiac etiology. TTM resulted in significantly reduced numbers of leukocytes in blood on days 3 and 5 after resuscitation illustrating a reduction in inflammation beyond the cooling period itself. A leukocyte count of less than 12.7 per microliter on day 3 undergoing TTM following CA significantly distinguished a better from a poor outcome in terms of survival during the first 30 days and long-term neurological performance.
CRP is another important marker of inflammation and it is known to be related to MI a long-term risk of death from cardiac causes in unstable coronary artery disease (37,38). In contrast to leukocyte levels, interestingly we observed a different effect of hypothermia on CRP in patients with CA due to cardiac origin. Despite divergent levels of this acute-phase protein were present on admission and ongoing cooling, its values aligned when normothermia was reached again.
Previous studies have shown that innate immunity has an important impact on healing after acute MI (8,9). In this context an elevated leukocyte count is an independent predictor for mortality after acute cardiac events (10,11). Immunosuppression by using steroids for example has shown mixed results in various trials (39). It has therefore been proposed that a balance is crucial as insufficient as well as exaggerated leukocyte presence may lead to impaired healing (28). In our current analysis we found that TTM suppresses leukocyte counts after CPR. So far, it remains unclear if hypothermia directly impacts leukocyte production and/or activation or if leukocyte counts are affected only indirectly by limitation of tissue damage. Future work is therefore needed to elucidate the underlying mechanism of the observed findings.
Limitations
As this study is a retrospective study, no randomization was performed or placebo control administered. Initiation of TTM was therefore the decision of the treating physician. This may have excluded patients with expected poor outcome from the TTM group and therefore be a possible selection bias. The finding that patients receiving TTM were in average about 10 years younger might also point in that direction. We addressed this limitation by performing logistic and Cox regression analyses. As leukocyte counts on day 3 and day 5 were further evaluated, patients dying in the very acute phase of the first 48 hours after admission are already naturally excluded at this point. Justified criticism could also be related to calculations of the cut-off level for leukocytes out of the all patients cohort. This was done because at the time of the manuscript there were not enough patients enrolled to calculate this in a reasonable manner for cardiac patients.
Nevertheless, this study underlines the beneficial effect of TTM following CA and emphasizes a possible role in influencing innate immunity and related outcome.
Acknowledgements
Funding: This work has been supported by the DZHK (German Centre for Cardiovascular Research). F Leuschner is supported by the German Research Foundation (DFG, LE 2530/2-1) and the BMBF (German Ministry of Education and Research; Project DeCaRe).
We thank the colleagues of the Institute of Medical Biometry and Informatics of the University of Heidelberg for fruitful discussions.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- WHO. Global status report on noncommunicable diseases 2014. Available online: http://www.who.int/nmh/publications/ncd-status-report-2014/en/
- Eisenberg MS, Mengert TJ. Cardiac resuscitation. N Engl J Med 2001;344:1304-13. [Crossref] [PubMed]
- Aune S, Eldh M, Engdahl J, et al. Improvement in the hospital organisation of CPR training and outcome after cardiac arrest in Sweden during a 10-year period. Resuscitation 2011;82:431-5. [Crossref] [PubMed]
- Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002;346:549-56. [Crossref] [PubMed]
- Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002;346:557-63. [Crossref] [PubMed]
- Perman SM, Grossestreuer AV, Wiebe DJ, et al. The utility of therapeutic hypothermia for post-cardiac arrest syndrome patients with an initial nonshockable rhythm. Circulation 2015;132:2146-51. [Crossref] [PubMed]
- Reichenbach DD, Moss NS, Meyer E. Pathology of the heart in sudden cardiac death. Am J Cardiol 1977;39:865-72. [Crossref] [PubMed]
- Kannel WB, Anderson K, Wilson PW. White blood cell count and cardiovascular disease. Insights from the Framingham Study. JAMA 1992;267:1253-6. [Crossref] [PubMed]
- Friedman GD, Klatsky AL, Siegelaub AB. The leukocyte count as a predictor of myocardial infarction. N Engl J Med 1974;290:1275-8. [Crossref] [PubMed]
- Furman MI, Becker RC, Yarzebski J, et al. Effect of elevated leukocyte count on in-hospital mortality following acute myocardial infarction. Am J Cardiol 1996;78:945-8. [Crossref] [PubMed]
- Palmerini T, Mehran R, Dangas G, et al. Impact of leukocyte count on mortality and bleeding in patients with myocardial infarction undergoing primary percutaneous coronary interventions: analysis from the Harmonizing Outcome with Revascularization and Stent in Acute Myocardial Infarction trial. Circulation 2011;123:2829-37, 7 p following 2837.
- Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 2013;339:161-6. [Crossref] [PubMed]
- Nahrendorf M, Swirski FK. Monocyte and macrophage heterogeneity in the heart. Circ Res 2013;112:1624-33. [Crossref] [PubMed]
- Leuschner F, Dutta P, Gorbatov R, et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol 2011;29:1005-10. [Crossref] [PubMed]
- Chakkarapani E, Davis J, Thoresen M. Therapeutic hypothermia delays the C-reactive protein response and suppresses white blood cell and platelet count in infants with neonatal encephalopathy. Arch Dis Child Fetal Neonatal Ed 2014;99:F458-63. [Crossref] [PubMed]
- Akriotis V, Biggar WD. The effects of hypothermia on neutrophil function in vitro. J Leukoc Biol 1985;37:51-61. [PubMed]
- Wenisch C, Narzt E, Sessler DI, et al. Mild intraoperative hypothermia reduces production of reactive oxygen intermediates by polymorphonuclear leukocytes. Anesth Analg 1996;82:810-6. [PubMed]
- Beilin B, Shavit Y, Razumovsky J, et al. Effects of mild perioperative hypothermia on cellular immune responses. Anesthesiology 1998;89:1133-40. [Crossref] [PubMed]
- Matsui T, Ishikawa T, Takeuchi H, et al. Mild hypothermia promotes pro-inflammatory cytokine production in monocytes. J Neurosurg Anesthesiol 2006;18:189-93. [Crossref] [PubMed]
- Matsui T, Ishikawa T, Takeuchi H, et al. Mild hypothermia inhibits IL-10 production in peripheral blood mononuclear cells. Acta Anaesthesiol Scand 2004;48:205-10. [Crossref] [PubMed]
- Ajam K, Gold LS, Beck SS, et al. Reliability of the Cerebral Performance Category to classify neurological status among survivors of ventricular fibrillation arrest: a cohort study. Scand J Trauma Resusc Emerg Med 2011;19:38. [Crossref] [PubMed]
- Geurts M, Macleod MR, Kollmar R, et al. Therapeutic hypothermia and the risk of infection: a systematic review and meta-analysis. Crit Care Med 2014;42:231-42. [Crossref] [PubMed]
- Callaway CW, Donnino MW, Fink EL, et al. Part 8: Post-Cardiac Arrest Care: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015;132:S465-82. [Crossref] [PubMed]
- Nolan JP, Soar J, Cariou A, et al. European resuscitation council and european society of intensive care medicine guidelines for post-resuscitation care 2015: section 5 of the European resuscitation council guidelines for resuscitation 2015. Resuscitation 2015;95:202-22. [Crossref] [PubMed]
- Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. N Engl J Med 2012;366:54-63. [Crossref] [PubMed]
- Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation 2013;127:143-52. [Crossref] [PubMed]
- Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med 2013;369:2197-206. [Crossref] [PubMed]
- Nahrendorf M, Pittet MJ, Swirski FK. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation 2010;121:2437-45. [Crossref] [PubMed]
- Nahrendorf M, Swirski FK. Innate immune cells in ischaemic heart disease: does myocardial infarction beget myocardial infarction? Eur Heart J 2016;37:868-72. [Crossref] [PubMed]
- Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol 2014;11:255-65. [Crossref] [PubMed]
- Dutta P, Courties G, Wei Y, et al. Myocardial infarction accelerates atherosclerosis. Nature 2012;487:325-9. [Crossref] [PubMed]
- Emami H, Singh P, MacNabb M, et al. Splenic metabolic activity predicts risk of future cardiovascular events: demonstration of a cardiosplenic axis in humans. JACC Cardiovasc Imaging 2015;8:121-30. [Crossref] [PubMed]
- Meybohm P, Gruenewald M, Albrecht M, et al. Hypothermia and postconditioning after cardiopulmonary resuscitation reduce cardiac dysfunction by modulating inflammation, apoptosis and remodeling. PLoS One 2009;4:e7588. [Crossref] [PubMed]
- Fries M, Stoppe C, Brücken D, et al. Influence of mild therapeutic hypothermia on the inflammatory response after successful resuscitation from cardiac arrest. J Crit Care 2009;24:453-7. [Crossref] [PubMed]
- Adrie C, Adib-Conquy M, Laurent I, et al. Successful cardiopulmonary resuscitation after cardiac arrest as a "sepsis-like" syndrome. Circulation 2002;106:562-8. [Crossref] [PubMed]
- Bro-Jeppesen J, Kjaergaard J, Wanscher M, et al. The inflammatory response after out-of-hospital cardiac arrest is not modified by targeted temperature management at 33 °C or 36 °C. Resuscitation 2014;85:1480-7. [Crossref] [PubMed]
- Lindahl B, Toss H, Siegbahn A, et al. Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease. FRISC Study Group. Fragmin during Instability in Coronary Artery Disease. N Engl J Med 2000;343:1139-47. [Crossref] [PubMed]
- Odeberg J, Freitag M, Forssell H, et al. Influence of pre-existing inflammation on the outcome of acute coronary syndrome: a cross-sectional study. BMJ Open 2016;6:e009968. [Crossref] [PubMed]
- Giugliano GR, Giugliano RP, Gibson CM, et al. Meta-analysis of corticosteroid treatment in acute myocardial infarction. Am J Cardiol 2003;91:1055-9. [Crossref] [PubMed]


