
Kinking of frozen elephant trunk: reality versus myth—a case report and literature reported
IntroductionOther Section
The Japanese made frozen elephant trunk (Frozenix®, Japan Lifeline, Tokyo, Figure 1) was first launched in the Japanese market in 2014. The case number of the Frozenix in 2014 was only 600 in Japan, however, the number has increased dramatically to 3,600 in 2020. Over 17,000 products have been used by the end of 2020. In the of 2014 or in 2015, the Frozenix was used for non-dissection aneurysm (NDTAA) in 50% and for acute type A aortic dissection (ATAAD) in 33% of patients. In 2020, this ratio was reversed to 51% in ATAAD and 41% in non-dissection TAA. As for ATAAD surgery in Japan, 55% of patients had replacement of ascending aorta or hemiarch, 30% had conventional total arch replacement (TAR), and 15% had TAR plus FET among 5,250 patients in 2016. In 2020, 7,000 patients had surgery for ATAAD and number of patients who had replacement of the ascending aorta or hemiarch decreased to 40% and that of TAR plus FET increased to 28%. Dominant sizes of the Frozenix used in ATAAD have been 23 to 29 mm in diameter and 9 or 12 cm in length (1).
Kinking or iatrogenic stenosis in the frozen elephant trunk (FET) is a possible complication and have been sporadically reported. In this report, our experience of this complication is presented, and possible mechanisms are discussed. The author presents the following case in accordance with CARE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-28/rc).
Case presentationOther Section
The patient was a 43-year-old male who was diagnosed to have an acute type A aortic dissection (ATAAD) in a local hospital and transferred to us 7 hours after onset of chest pain. His consciousness was clear and all arterial pulses were palpable. There was a small pericardial effusion and aortic regurgitation was minimum by echocardiography. The CT scan showed that he had DeBakey type I dissection and that the false lumen was not thrombosed. There was a big entry tear at the level of the left subclavian artery and the true lumen was narrowed at the aortic bifurcation.
The cardiopulmonary bypass was started using the femoral cannulation and he was cooled to 23 ℃. The patient was positioned to the Trendelenburg position, and the ascending aorta and aortic arch was incised after circulatory arrest and the heart was arrest using the retrograde cardioplegia. The antegrade cerebral perfusion was started using three cannulas inserted to the arch vessels from inside the arch. The aorta was transected distal to the intimal tear at the level of the left subclavian artery and a frozen elephant trunk (FET; Frozenix® 23 mm × 6 cm, Japan Lifeline, Tokyo) was inserted in the true lumen of the descending aorta. The FET was fixed with an outer Teflon felt strips with three 5-0 monofilament mattress sutures. A four branched Dacron graft (J graft® 26 mm, Japan Lifeline, Tokyo) was anastomosed using a continuous 4-0 monofilament suture to the proximal stump of the descending aorta including the FET. Then, distal perfusion was resumed and the patient was rewarmed. Proximal anastomosis was done at the level of the ST junction of the ascending aorta. Three aortic valve commissures were resuspended and an inner strip of the Dacron graft and an outer Teflon felt were used to reinforce the anastomosis. Then, coronary perfusion was re-started, and the heart was defibrillated. Three arch vessels were anastomosis using the button technique to the graft branches. Duration of the CPB was 237 minutes, cardiac ischemic time 117 minutes, duration of the circulatory arrest of the lower body 62 minutes, duration of the ACP 181 minutes. The minimum temperature of the tympanic membrane and rectum was 22.1 and 28.2 ℃, respectively. The patient weaned the bypass without any difficulty, however, the femoral pulses were not palpable and there was no urine production. A right axillary artery to bilateral femoral extra-anatomical bypass was made using a 8 mm ringed Gore-Tex graft. On the 3rd postoperative day, an aortography and CT scan showed a severe stenosis at the non-stented portion of the FET (Figure 2). The pressure gradient at the stenotic portion was 100 mmHg and there was a leakage to the false lumen. An endovascular stent-graft (TX-D® 28 mm. Zenith Cook) was used to relieve the stenosis (Figure 3). He was discharged and back to the normal life. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.

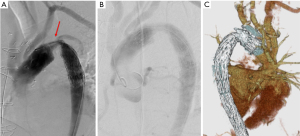
DiscussionOther Section
In 1983, Borst et al. introduced the “free” elephant trunk principle into the surgical strategy for an extensive thoracic aortic disease (2). In 1996, Kato et al. (3) first reported 10 patients who had undergone home-made stent-graft insertion in the descending aorta, and they named this method “open stent grafting”. In 2003, Karck et al. (4) reported 4 patients who had an open aortic arch replacement with stent-graft insertion on the descending aorta. This was the first report where this method was called “frozen elephant trunk (FET)”. Since then, the frozen elephant trunk procedure has been widely spread mainly in Europe and many clinical investigations have been reported using commercial-made products (5-10).
Japan-made FET, Frozenix, was launched 2014 and since then, more than 17,000 prostheses have been implanted. The Nitinol stent was hand-knitted not to change length as the diameter change. All stents were fully covered with the Dacron prosthesis. The proximal portion of the Frozenix stent graft was composed of a simple Dacron graft. The Dacron graft had external velour with a thickness of 300 micro mm and its water porosity was 150 mL/cm2. The delivery system consisted with a malleable rod (10 Fr) and could be advanced into the descending aorta over a 0.035-inch flexible guide wire the stent-graft. The system was wrapped by a smooth-surfaced polyester mesh. The sheath had markers of 1 cm-interval in the non-stented portion and the last marker should be pointed at the edge of distal aorta. Because the stent did not change its length throughout, the distal end of the stent graft can be fixed as expected. Withdrawal of the outer sheath while the inner rod was held steady released the stented portion of the Frozenix stent graft. The proximal Dacron tube could then be released by simply pulling back both the sheaths and rod. The products had a range of diameter from 21 to 39 mm (2 mm step) and the length of the stented portion was 60 mm, 90 mm, 120 mm, and 150 mm. The total length of the stent graft was 200 mm in all. The length of the total system was 57 cm and 12 mm in diameter (Figure 1).
Main advantages of usage of the FET in patients with ATAAD is to facilitate distal anastomosis in zone II or in zone III. In addition, bleeding from the distal anastomosis is seldom seen, and there is less incidence of leakage to the distal false lumen occurs, resulting in positive remodeling of the distal aorta. Some of the patients who had FET at the first stage had the subsequent endovascular stent-graft insertion in the descending aorta. We conducted a multi-institutional study dealing with FET usage from 2016 to 2018, and there were 154 patients with ATAAD underwent total arch replacement plus FET insertion. Mean ages were 61.1 years and hospital death occurred in 3 patients (1.9%), permanent stroke in 12 (7.8%), and paraplegia in 4 (2.6%) (11,12). The annual survey of Japanese Association of Thoracic Surgery found a steep increase of usage of the FET in surgical strategy for the ATAAD. In 2016, patients with ATAAD who had total arch replacement plus FET consisted of 15% in whole 5,250 patients who had surgery for the ATAAD and that became 28% of 7,000 patients with ATAAD in 2020 (1). Subsequently, ratio of the total arch replacement in total ATAAD patients in Japan increased from 49% in 2016 to 60% in 2020.
Several complications regarding with the FET usage have been recognized. The main concern is a relatively higher incidence of development of spinal cord ischemia. The incidence was not negligible and has remained constant. Deliberate deployment of the distal stent-graft in the descending aorta above the level of the aortic valve should be warranted to prevent spinal cord complications (11). Also, exclusion of the patients with shaggy descending aorta is recommended. Distal stent-induced new entry (DSINE) has been reported to occur in 5% to 7% in FET usage and DSINE has occurred more frequently in routine TEVAR (thoracic endovascular aortic repair) procedures in patients with type B aortic dissection (13). Placing the FET in the vertical position in the true lumen of descending aorta and avoidance of over-sizing the FET relative to the diameter of the descending aorta was effective.
Kinking of the FET or stenosis have been reported on several occasions, resulting in the low perfusion status of the lower body and proximal hypertension. The reports have been published in series of case reports and there was no collective reviews focusing on this complication (14-22) (Table 1). Majority of the patients who had the FET stenosis showed that the junction of the stented portion and non-stented portion of the FET was placed at the steep angle of the aortic arch. Taguchi et al. (20) warned a risk of kinking of the FET when it was used in patients with right aortic arch because that there is steep angle in the distal arch in this setting. Most reports warned that the length of the non-stented portion was too long. Especially the FET was used in non-dissection aneurysm cases who had zone II distal anastomosis where a large space was remained around the FET. Sometimes, they had anastomotic leakage (distal anastomotic new entry; DANE) to the false lumen of the descending aorta (Figure 3), which may cause a pressurization of false lumen of the descending aorta. In most cases, this stenosis was corrected by an additional stent-graft insertion from the femoral artery. Deschka et al. (22) used a bare stent. Alternatively, Uchida et al. (15) re-anastomosed the proximal portion of the FET and distal portion. Taguchi et al., and we performed an extra-anatomical bypass to the lower body.
Table 1
| Authors | City | Year | Case | Lesions | Procedure | Distal anastomosis | DANE | FET | Secondary procedure | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Easo | Oldenburg | 2007 | 53 male | CTAAD s/p Hemiarch | TAR | Chavan-Haverich | TEVAR | Alive | ||
| Pacini | Bologna | 2008 | 52 male | CTAAD Marfan, s/p MVR, Scoliosis | TAR, Bentall | Zone III | Evita | TEVAR | Alive | |
| Nakao | Tokyo | 2013 | 49 male | CTBAD, Kommerell div | TAR | Zone II | Gore-Tag | TEVAR | Alive | |
| Deschka | Munster | 2014 | 51 male | ATAAD | TAR, Freestyle root | Zone III | Evita | Palmaz stent | Alive | |
| Present case | Kobe | 2015 | 43 male | ATAAD | TAR | Zone III | Yes | Frozenix | Ax-FA bypass, TEVAR | Alive |
| Motomatsu | Fukuoka | 2016 | 72 male | TAA, distal arch | TAR | Zone II | Frozenix | TEVAR | Alive | |
| Wakiyama | Kobe | 2017 | 76 male | ATAAD retrograde | TAR | Zone II | Frozenix | TEVAR | Alive | |
| Taguchi | Hirosaki | 2018 | 67 male | ATAAD, RAA, Kommerell div | TAR | Zone III | Frozenix | Ax-FA bypass | Alive | |
| Morizaki | Osaka | 2019 | 44 male | ATAAD | TAR, VSRR | Zone II | Frozenix | TEVAR | Alive | |
| 30 male | ATAAD, AAE, Marfan | TAR, VSRR | Zone II | Frozenix | TEVAR | Alive | ||||
| Uchida | Yamagata | 2019 | 66 male | CTAAD, TAA distal arch | TAR | Zone II | Frozenix | Re-anastomosis | Alive |
s/p, status post-operation; DANE, distal anastomosis new entry; FET, frozen elephant trunk; ATAAD, acute type A aortic dissection; CTAAD, chronic type A aortic dissection; CTBAD, chronic type B aortic dissection; TAA, non-dissection thoracic aorta aneurysm; MVR, mitral valve replacement; RAA, right aortic arch; div, diverticulum; AAE, annuloaortic ectasia; TAR, total arch replacement; VSRR, valve sparing aortic root replacement; TEVAR, thoracic endovascular aneurysm repair; Ax-FA, axillo-femoral.
Surgeons should be aware of the arch topology, such as the size, direction of the blood stream, and morphology preoperatively and should try to place the stented portion of the FET in the aortic arch angle. Also, we should make the non-stented portion as short as possible at the distal anastomosis to prevent FET kinking. The DANE may cause the pressurization of the distal false lumen and should be avoided by the secure and meticulous techniques. Over-sizing or under-sizing of the FET should be minimized because the FET may cause DSINE and migrated distally (23). Alternatively, Yamamoto et al recommended “zone 0 arch repair” to facilitate distal anastomosis in patients with ATAAD (24).
ConclusionsOther Section
To prevent the FET kinking, surgeons should place the stented portion of the FET in the aortic arch angle. Also, we should make the non-stented portion as short as possible at the distal anastomosis. The DANE (distal anastomosis new entry) should be avoided by the secure anastomosis. Over-sizing or under-sizing of the FET should be minimized.
AcknowledgmentsOther Section
Funding: None.
FootnoteOther Section
Provenance and Peer Review: This article was commissioned by the Guest Editors (Mohamad Bashir, Mohammed Idhrees and Edward P. Chen) for the series “Frozen Elephant Trunk” published in Cardiovascular Diagnosis and Therapy. The article has undergone external peer review.
Reporting Checklist: The author has completed the CARE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-28/rc
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-28//coif). The series “Frozen Elephant Trunk” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery. Thoracic and cardiovascular surgeries in Japan during 2018: Annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2021;69:179-212. [Crossref] [PubMed]
- Borst HG, Walterbusch G, Schaps D. Extensive aortic replacement using "elephant trunk" prosthesis. Thorac Cardiovasc Surg 1983;31:37-40. [Crossref] [PubMed]
- Kato M, Ohnishi K, Kaneko M, et al. New graft-implanting method for thoracic aortic aneurysm or dissection with a stented graft. Circulation 1996;94:II188-93. [PubMed]
- Karck M, Chavan A, Hagl C, et al. The frozen elephant trunk technique: a new treatment for thoracic aortic aneurysms. J Thorac Cardiovasc Surg 2003;125:1550-3. [Crossref] [PubMed]
- Usui A, Fujimoto K, Ishiguchi T, et al. Cerebrospinal dysfunction after endovascular stent-grafting via a median sternotomy: the frozen elephant trunk procedure. Ann Thorac Surg 2002;74:S1821-4; discussion S1825-32. [Crossref] [PubMed]
- Di Bartolomeo R, Di Marco L, Armaro A, et al. Treatment of complex disease of the thoracic aorta: the frozen elephant trunk technique with the E-vita open prosthesis. Eur J Cardiothorac Surg 2009;35:671-5; discussion 675-6. [Crossref] [PubMed]
- Jakob H, Dohle D, Benedik J, et al. Long-term experience with the E-vita Open hybrid graft in complex thoracic aortic disease. Eur J Cardiothorac Surg 2017;51:329-38. [Crossref] [PubMed]
- Leontyev S, Tsagakis K, Pacini D, et al. Impact of clinical factors and surgical techniques on early outcome of patients treated with frozen elephant trunk technique by using EVITA open stent-graft: results of a multicentre study. Eur J Cardiothorac Surg 2016;49:660-6. [Crossref] [PubMed]
- Luehr M, Etz CD, Mohr FW, et al. Surgical management after stent-graft failure during the frozen elephant trunk technique for acute type A aortic dissection. J Thorac Cardiovasc Surg 2012;144:e106-8. [Crossref] [PubMed]
- Tian DH, Wan B, Di Eusanio M, et al. A systematic review and meta-analysis on the safety and efficacy of the frozen elephant trunk technique in aortic arch surgery. Ann Cardiothorac Surg 2013;2:581-91. [PubMed]
- Ogino H, Okita Y, Uchida N, et al. Comparative study of Japanese frozen elephant trunk device for open aortic arch repairs. J Thorac Cardiovasc Surg 2021; [Epub ahead of print]. [Crossref] [PubMed]
- Okita Y, Kumamaru H, Motomura N, et al. Current status of open surgery for acute type A aortic dissection in Japan. J Thorac Cardiovasc Surg 2020; [Epub ahead of print]. [PubMed]
- Czerny M, Eggebrecht H, Rousseau H, et al. Distal Stent Graft-Induced New Entry After TEVAR or FET: Insights Into a New Disease From EuREC. Ann Thorac Surg 2020;110:1494-500. [Crossref] [PubMed]
- Pacini D, Armaro A, Di Marco L, et al. Stent graft coarctation after frozen elephant trunk procedure: an unusual complication. J Thorac Cardiovasc Surg 2009;137:1027-9, 1029e1.
- Uchida T, Kuroda Y, Yamashita A, et al. Unexpected intraoperative obstruction of frozen elephant trunk in patients who underwent total arch replacement. J Card Surg 2019;34:1673-5. [Crossref] [PubMed]
- Morisaki A, Isomura T, Fukada Y, et al. Kinking of an open stent graft after total arch replacement with the frozen elephant technique for acute Type A aortic dissection. Interact Cardiovasc Thorac Surg 2018;26:875-7. [Crossref] [PubMed]
- Easo J, Dapunt O, Natour E, et al. Transfemoral stent-graft placement to treat a complication of the frozen elephant trunk procedure. J Endovasc Ther 2007;14:260-3. [Crossref] [PubMed]
- Motomatsu Y, Oishi Y, Matsunaga S, et al. Endovascular aortic repair immediately after open stent-grafting: collapse of the endograft. Eur J Cardiothorac Surg 2017;51:390-2. [PubMed]
- Nakao M, Yamashiro M, Matsumura Y, et al. Lower body ischemia due to bending of the stent after hybrid treatment for chronic Stanford type B aortic dissection. Kyobu Geka 2013;66:791-4. [PubMed]
- Taguchi R, Kowatari R, Minakawa M, et al. Open Stent Graft Stenosis after Total Arch Replacement for Acute Aortic Dissection in a Patient with Right-sided Aortic Arch. Kyobu Geka 2018;71:1068-72. [PubMed]
- Wakiyama E, Obo H, Izumi S, et al. Emergency total arch replacement with J-graft open stent graft for acute type A aortic dissection requiring TEVAR for stent graft stenosis. Jpn J Cardiovasc Surg 2017;46:38-44. [Crossref]
- Deschka H, Nolte T, Machner M, et al. Distortion of a hybrid stent graft following a frozen elephant trunk procedure. J Card Surg 2014;29:650-2. [Crossref] [PubMed]
- Kandola S, Abdulsalam A, Field M, et al. Frozen elephant trunk repair of aortic aneurysms: How to reduce the incidence of endoleak and reintervention. JTCVS Tech 2020;3:13-20. [Crossref] [PubMed]
- Yamamoto H, Kadohama T, Yamaura G, et al. Total arch repair with frozen elephant trunk using the "zone 0 arch repair" strategy for type A acute aortic dissection. J Thorac Cardiovasc Surg 2019; Epub ahead of print. [Crossref] [PubMed]




