
A narrative review of the surgical management of Paget-Schroetter syndrome: case series and long-term follow-up
Introduction
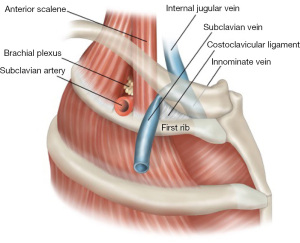
Paget-Schroetter syndrome (PSS) is a thrombosis of the axillary-subclavian vein (SV), due to repetitive use of the arm associated with ‘the presence of one or more compressive elements in the thoracic outlet’ (1). The condition mainly affects young males and is relatively uncommon for those with thoracic outlet syndrome, accounting for 1–4% of all cases of venous thrombosis. The incidence of PSS is estimated to be between 1 to 2 per 100,000 population (2). The SV is the most anterior structure as it traverses the first rib from medial to lateral from the superior mediastinum.
The first rib is the most curved and usually the shortest and furthermore it is broad and flat. The head of the rib is small and rounded whilst the neck is narrow with the tubercle on the outer border. The upper surface has two shallow grooves with a ridge between them and is prominent medially as a bony protuberance. This is the scalene tubercle where the scalenus anterior muscle inserts. The anterior groove is where the SV runs from the thoracic cavity laterally into the arm whilst the subclavian artery and the lowest trunk of the brachial plexus runs in the posterior groove (Figure 1).
Apart from surrounding anatomical structures, intrinsic vascular factors exist that may dramatically increase the risk of PSS occurring. Chronic stenosis of the SV resulting from repeated motion-induced compression and paraneoplastic pro-thrombotic states are examples of documented intrinsic lesions that impede flow in the SV independent of surrounding structures, and lead to PSS (3).
There is evidence anticoagulation alone may not be sufficient to reduce the incidence of post-thrombotic syndrome and open surgery is required in addition to repair the anatomical defect (4). The current optimum management for PSS is immediate catheter-directed thrombolysis (CDTL) followed by surgical decompression of the SV by first rib resection (FRR) (5,6). Proceeding with both endovascular and open surgical interventions ensures that intrinsic and extrinsic insults are treated and may help to further mitigate the risk of recurrent thromboses—the combined approach has been noted to allow 100% of patients to regain preoperative function in the affected upper limb (5).
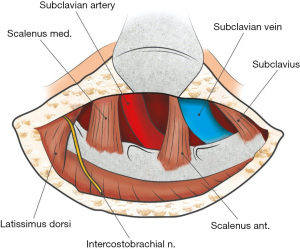
Successful extravascular management of PSS depends on a thorough understanding of the anatomical structures that may impinge on the SV and cause extrinsic compression. The subclavius muscle and tendon are located medially at the thoracic outlet and can potentially cause nutcracker-like compression of the SV with very minimal movement (Figures 1,2). The costoclavicular ligament (CCL) inserts inferiorly to the upper medial aspect of the cartilage of the first rib and proceeds posterolaterally to the costal tuberosity on the inferior aspect of the medial clavicle. Rigberg in 2006 described the ‘scissoring’ effect between the clavicle and first rib with arm movement, to explain the potential for compression of neurovascular structures at the thoracic outlet (7).
This paper seeks to evaluate the roles of endovascular intervention in addition to FRR for the management of PSS. The existing literature was reviewed to determine the significance of adopting both intervention modalities for the successful treatment of PSS. In addition, data gathered over 15 years in a tertiary referral centre in South Wales concerning the surgical approaches for FRR is included. The outcomes scrutinised after thrombolysis, surgical decompression and venoplasty included primary and secondary SV patency rates, adjunctive measures performed on the initial admission and symptom relief gained. We present the paper in accordance with the Narrative Review reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-158/rc).
Methods
Our narrative review of current therapeutic approaches to PSS was carried out in accordance with the search strategy summary summarised in Table 1.
Table 1
| Items | Description |
|---|---|
| Date of search | July 2021 |
| Databases searched | PubMed, Embase, Web of Science |
| MeSH search terms used | Paget-Schroetter Syndrome |
| Endovascular repair | |
| Open surgical repair | |
| Medical treatment | |
| Catheter-directed thrombolysis | |
| Surgical approach | |
| Clinical outcomes | |
| Search timeframe | 01 January 2000–July 2021 |
| Selection process | Relevant titles were collated by SZT and MJ. Abstracts were vetted through our inclusion and exclusion criteria by SZT and MJ. A final list of abstracts was circulated between all authors to ensure consensus on the selected studies |
| Inclusion criteria | Content |
| Studies involving adult humans with PSS | |
| Studies discussing therapeutic strategies for PSS | |
| Clinical outcomes associated with PSS | |
| Language | |
| Studies in English | |
| Study type | |
| Original articles | |
| Literature reviews | |
| Systematic reviews | |
| Commentaries |
PSS, Paget-Schroetter syndrome.
What role does endovascular intervention play in managing PSS?
Once the diagnosis of PSS is confirmed via history, physical examination and imaging (such as X-ray of the thoracic inlet, duplex ultrasonography, or venography) treatment typically proceeds in several stages, commencing with thrombolysis, followed by surgical decompression, and finally adjunctive angioplasty or possibly venous bypass in certain cases (5).
A hybrid endovascular/open decompression approach to treat PSS is not always felt to be necessary. Depending on local guidelines, patient characteristics, and myriad factors, the managing clinician may opt to proceed with endovascular intervention or even only open decompression. An approach employing both endovascular techniques alongside open decompression is associated with excellent technical success rates, and event-free survival (8). However, due to proximity of vital structures neurological sequelae may occur and even inadvertent removal of the second rib during surgery (9). Zurkiya et al. (10) emphasise that timely endovascular lysis or debulking of the thrombus is a well-documented contributing factor to the preservation of long-term luminal patency, and to the mitigation of re-occlusion, scarring, and intimal fibrosis. Further, Koury et al. (11) suggests that FRR may not be required if complete lysis is achieved via thrombolysis and there remains no evidence of residual stenosis or re-thrombosis.
The endovascular management of PSS is principally centred on either CDTL or endovascular thrombectomy. Koury et al. (11) describe the surgical technique for CDTL in upper extremity deep vein thrombosis (UEDVT) as beginning with gaining venous access in the ipsilateral upper extremity. Venography is carried out to determine the extent of thrombosis within the SV. If thrombolysis is commenced a guidewire is crossed over the lesion, to allow the infusion catheter to be positioned as near the thrombus as possible. Thrombolytic agents such as tissue plasminogen activator (tPA) or urokinase are then infused for a duration of at least 8 hours, or in some cases up to 72 hours within a high dependency unit. Patient fibrinogen levels and SV patency are monitored continuously. Catheter-related thrombosis can be minimised via the concurrent infusion of a subtherapeutic dose of heparin. Koury et al. (11) further note that CDTL is often augmented with mechanical thrombectomy, angioplasty, or even endovascular stent placement to correct factors such as underlying stenosis.
Landry et al. (5) note that CDTL may be associated with prolonged treatment durations and adverse events including cerebral haemorrhage, pulmonary embolism, and access site bleeding may occur. As a result, mechanical thrombectomy using devices such as the AngioJet (Boston Scientific, USA) used with the power pulse spray technique are being increasingly employed to treat PSS [as opposed to iliofemoral deep vein thrombosis (DVT)].
The AngioJet utilises a perforated catheter tip through which high-velocity, high-pressure streams of saline are projected. The resulting local region of low pressure allows entrapment and retrieval of bulky thrombi via the Venturi-Bernoulli effect (12,13). Though this system has historically been used more extensively for thrombus debulking in acute coronary syndromes and iliofemoral DVTs, Schneider and colleagues suggest the use of the AngioJet system in the setting of PSS is promising—they report an average treatment time of 12 hours overall, a significant reduction in comparison to that of CDTL (14).
Completion of thrombolysis via endovascular intervention is then typically followed by surgical decompression of surrounding anatomical structures, before further adjunctive endovascular measures are implemented. Aggressive angioplasty prior to surgical decompression is generally discouraged, to prevent inflicting barotrauma to the wall of the SV while under anatomical compression.
Surgical resolution of extrinsic venous compression
FRR has historically, usually been performed via the transaxillary (TA) or supraclavicular (SC) approach. The SC approach provides excellent access for surgical decompression of the brachial plexus and subclavian artery but can be difficult to access the SV due to its location anterior to the scalene muscles and posterior to the manubrium. For this reason, a paraclavicular (PC) or an infraclavicular (IC) incision has been considered an alternative option for successful surgical management specifically of PSS (2).
Surgical approaches
TA approach—Figure 3
This requires meticulous preparation with the patient prepped to enable access via a 10 cm horizontal skin incision over the third rib. The lateral aspect of the pectoralis major and anterior border of the latissimus dorsi should be exposed. This incision exposes the fascia of the serratus anterior enabling the dissection to proceed proximally towards the apex of the axilla. Upward traction on the arm at this stage enables the scalenus anterior to be identified with the SV and artery either side. The insertion of the scalenus anterior into the first rib can then be divided. This then exposes the anterior aspect of the first rib with the subclavius muscle visible under the head of the clavicle. This also needs to be divided at its origin from the medial aspect of the first rib taking care not to damage the SV. Inferiorly, the intercostal muscles are divided from the lateral aspect of the first rib which enables the pleura to be dissected free from the inferior aspect of the rib. The first rib can then be grasped and divided at the costochondral junction and divided as far posteriorly as necessary to isolate the SV and remove any extrinsic bands. The critical technical steps for successful decompression by the TA approach are excision of the anteriorly placed subclavius muscle and circumferential periadventitial dissection of the SV.
IC approach—Figure 4
The incision commences 2 cm below the clavicle and extends medially to the sternal border. The two origins of the pectoralis major muscle are from the anterior surface of the sternum (sternocostal part) and anterior surface of medial half of the clavicle (clavicular part). Between these two origins a non-muscle splitting approach enables the SV to be identified. The principal advantage of the IC approach is that an incision is made over the first rib in order to access the anteriorly located SV. Removal of this part of the first rib is crucial in the satisfactory treatment of SV thrombosis as direct exposure of the anteromedial aspect of the SV is provided. This enables rigorous debulking of the costochondral junction and subclavius muscle resection from the origin at the superior aspect of the medial first rib. The intercostal muscles can be divided from the lateral rounded aspect of the first rib and medially the dissection can proceed posteriorly to divide the insertion of the scalenus anterior muscle. This incision should only be used when the SV needs to be decompressed as access to the artery and plexus are better served via the TA or with a separate SC incision. The IC incision can be combined with an SC approach—the PC approach. This enables a more complete FRR to be performed than a TA or SC approach. Furthermore, as the medial aspect of the SV is directly exposed, good access for any reconstruction is easily provided.
Postoperative management
Following FRR, antiplatelet and/or anticoagulation therapy is invariably instigated, follow-up investigations include doppler ultrasound and venography to determine venous luminal patency in terms of blood flow at the lesion site, and to detect any residual stenosis or re-thrombosis that may require reintervention (15).
Because thoracic outlet decompression in PSS fails to address intrinsic vessel wall lesions that may arise secondary to chronic anatomical compression, residual SV lesions with a stenosis or recurrent thrombosis remain a particularly distinct cause for reintervention (4). In cases where persistent venous stenosis is detected on postoperative imaging, adjunctive percutaneous transluminal angioplasty (PTA) or more controversially stenting is a well-documented approach to improve long-term clinical outcomes and maintain the re-establishment of the native luminal diameter (5).
Schneider et al. note that up to 60% of patients persistently exhibit recurrent thrombosis or SV stenosis following surgical decompression, and that adjunctive angioplasty in this setting was found to be highly effective. Indeed, fibroelastic venous wall lesions in patients with PSS may necessitate balloon inflation pressures exceeding 10 atmospheres (atm) to achieve successful venous dilation (3,14). Re-thrombosis of the SV lumen often occurs in the interval between decompressive surgery and adjunctive intervention, especially in cases where preoperative endovascular thrombolysis was not carried out, or full thrombolysis was not achieved. This has led surgeons to advocate for performing endovascular thrombolysis, decompression, and adjunctive procedures within a single hospitalization (3). This approach would arguably speed up recovery time, and shorten the overall duration of admission and treatment. As an alternative, Koury et al. (11) suggest prophylactic SVC filter placement prior to decompressive surgery, in cases where endovascular or systemic thrombolysis has failed or is contraindicated, especially when there is a significant risk of thromboembolic risk. Placement of such a filter at the confluence of the left and right innominate vein would therefore protect against cerebral, azygous, or pulmonary embolization.
It must be stressed, however, that adjunctive angioplasty or endovascular stenting does not serve to replace decompressive surgery in the surgical management of PSS. Schneider concluded venoplasty alone for PSS in those who have not undergone decompressive surgery is particularly ineffective (3). Though lumen patency may temporarily be maintained by a stent, anatomical compression during movement may easily result in stent fracture and SV re-thrombosis adjunctive stenting is less effective in patients with chronic PSS because well-established thrombi typically respond poorly to balloon dilation as well as endovascular thrombolysis.
How does the surgical approach to decompression affect clinical outcome?
The following is derived from a retrospective review of patients presenting with acute PSS and undergoing open decompressive surgery at the University Hospital of Wales. The purpose is the evaluation of hybrid treatment approaches (involving endovascular intervention and adjunctive therapy) for PSS.
Clinical series methodology
Only two vascular surgeons (IMW/RJW) routinely perform thoracic outlet decompressive surgery at University Hospital of Wales for PSS. Hence, all patients treated surgically by them for PSS were included in the series. All presenting with a suspected diagnosis of PSS underwent complete history and examination followed by X-ray of the thoracic inlet and duplex ultrasonography to confirm the diagnosis. If thrombus was detected in the SV and there were no contraindications, a full discussion was held with the patient regarding thrombolysis. The risks of thrombolysis were explained as were the potential benefits. It is unit policy to offer open surgical decompression following thrombolysis at the same admission.
The selection of patients to undergo open surgical decompression was down to each individual surgeon and considered several factors. These included the age, whether it is the dominant arm affected, the duration of the thrombus within the SV, and acceptance of risks of lysis followed by open decompressive surgery. There is evidence to support surgical treatment of PSS as if treated medically (anticoagulation/antiplatelets) there is a risk of developing a post phlebitic limb and associated morbidity in 7–46%, mean 15% of cases (16). A flow chart representing the treatment plan for managing PSS is shown in Figure 5.
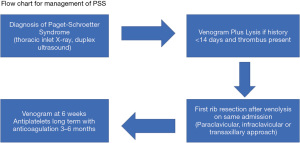
Patients were initially treated with CDTL using tPA via the ipsilateral cephalic vein, at 1 mL/hr after a 10 mg bolus dose. This was repeated after 6 hours with a further venogram performed within 24 hours. tPA was either continued (for 6 further hours) or discontinued, depending on the residual thrombus load within the SV. Surgical decompression was then carried out in accordance with the IC, TA, and SC routes outlined previously, and all patients underwent FRR under general anaesthetic. The surgical approach was decided by each surgeon on an individual basis. Patients were then commenced on antiplatelet therapy (aspirin) combined with 3–6 months of anticoagulation and latterly rivaroxaban. This was then stopped and antiplatelets were continued long-term. Venography was performed at 6 weeks post-surgery to assess patency and to treat residual stenosis if present.
Results (Table 2)
Table 2
| Demographics | Values |
|---|---|
| Male, (n) | 15 |
| Female, (n) | 11 |
| Age, median [range], years | 37 [19–52] |
| Duplex ultrasound, (n) | 26 |
| Venogram, (n) | 26 |
| Preoperative lysis + venoplasty, (n) | 5 |
| Postoperative venoplasty, (n) | 6 |
| Time to present, median [range], days | 4 [2–30] |
| Lysis time, median[range], hours | 22 [6–46] |
| Surgical approach, (n) | |
| PC | 4 |
| IC | 12 |
| TA | 9 |
| SC | 1 |
| Follow-up, median [range], months | 36 [7–168] |
| Secondary patency rates SV, (n) | 23/26 |
| Symptom resolution, (n) | 26 |
PC, paraclavicular; IC, infraclavicular; TA, transaxillary; SC, supraclavicular.
Twenty-six underwent FRR for SV obstruction over a 15-year time period [2005–2020] with eleven (42%) female and 15 (58%) male. Median range for length of time of symptoms was 4 [2–30] days whilst length of time of tPA lysis was 22 [6–46] hours. One patient had a free floating first rib and three others had bony abnormalities related to the medial clavicle and costoclavicular junction (Figures 6-9).
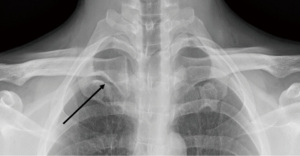
Three (12%) had no intraluminal clot on initial duplex ultrasonography and did not receive thrombolysis. Of these, 2 presented >30 days post initial symptoms and had a stenosis in the SV whilst the third underwent revision surgery due to inadvertent second rib resection via the TA approach for PSS at another vascular institute.
Of the 23 (88%) receiving lysis all underwent FRR. Overall, 12 (46%) underwent FRR by the IC approach with 9 (35%) by the TA route. Four (15%) underwent a PC approach and one by the SC route. Sixteen (72%) had a tight stenosis in the SV at the medial aspect of the first rib. Furthermore, 2 of these stenoses were greater than 2 cm in length. A further 3 were found to have a moderate SV stenosis after lysis and the remaining one an occlusion. Eleven venoplasties of the SV were performed—5 prior to surgical decompression and 6 post. Follow up was for a median [range] of 36 [7–168] months and all 26 remained asymptomatic clinically with no evidence of post thrombotic sequelae in the affected arm.
Outcomes
Three SVs occluded following FRR (1 TA, 1 PC and 1 IC) at 36, 4 months and 1 day post decompression. Of these, 2 (TA and PC) underwent SV venoplasty which was successful in the first patient but in the second the SV occlusion was unable to be crossed with a wire. This was treated conservatively with anticoagulation as was the patient who occluded the SV one day post decompression via the IC approach. All 3 remained asymptomatic with the secondary patency rates of SV 23/26 (88%).
Symptom resolution
Twenty-three had a patent SV on follow-up. This included a patient who reoccluded after 36 months and presented with a symptomatic upper limb venous thrombosis. This was treated with lysis (no venoplasty) and the SV remains patent on long term anticoagulants. Two remain patent but with scarred post thrombotic SVs.
Discussion
Optimum surgical treatment of PSS nowadays requires both endovascular intervention and adequate surgical decompression of the SV from surrounding extrinsic structures. The clinical data from this series has shown that the majority undergoing thrombolysis and decompression had a definite venous wall stenosis secondary to longstanding extrinsic compression. Forty-two percent (n=11) underwent venoplasty either pre- or post-surgical decompression. The durability of open surgery is impressive where adequate venolysis or early venoplasty maintain patency of the SV and reduce rates of recurrent stenosis. Secondary patency rates were 88% (23/26) at a median follow-up of 36 months. All of the cohort were asymptomatic despite 3 patients occluding the SV post-surgery. Reasons SV occlusion can be asymptomatic may possibly be explained by venous collateralisation. However, it is uncertain whether FRR actually improves collateralisation when the SV is chronically occluded. For this reason, the benefits of decompressive surgery for a chronically or subacutely thrombosed SV might reasonably be questioned.
Three did not undergo CDTL with 2 presenting more than 30 days after initial symptoms of PSS and both undergoing FRR. They must be differentiated from McCleery syndrome which is a distinct entity as SV compression occurs in the absence of SV luminal thrombus. Details on the optimum management for this condition are few but there are reports of successful FRR being performed with good long-term patency rates and remaining asymptomatic (17,18).
All patients in the cohort underwent thrombolysis via CDTL only and is associated with several clinical limitations. These include a possibly prolonged treatment time and certain adverse events including pulmonary embolism and entry site bleeding (5). Review of the current literature suggests that alternatives to CDTL for the management of PSS are available (Table 3). Schneider et al. (14) reported an average thrombolysis time of 12 hours overall (compared to 22 hours in the included series) when the AngioJet (Boston Scientific) mechanical thrombolysis system was used for thrombus debulking prior to CDTL. Similarly, Shah et al. (19) reported that use of the AngioJet system yielded successful thrombolysis in 2–3 hours for three patients. Hileman proposed mechanical thrombectomy as an alternative to CDTL in PSS where 93% of patients showed >50% clot reduction. compared to 79% of patients treated with CDTL only (20). Furthermore, O’Sullivan reported an average thrombolysis time of 91 minutes using the Trellis thrombolysis catheter (Covidien) (21). This alternative to traditional CDTL, also conventionally used for treating lower extremity DVT (LEDVT), was associated with 50–95% clot removal in 82% of patients, and >95% removal in 3 patients. It was also associated with no major complications.
Table 3
| Study | Year | Intervention | Clinical outcomes |
|---|---|---|---|
| Schneider et al. (14) | 2003 | AngioJet debulking followed by CDTL | • Average thrombolysis time of 12 hours overall |
| • 60% of patients found to have post-decompression residual stenosis, successfully managed via PTA | |||
| O’Sullivan et al. (21) | 2007 | Trellis catheter followed by surgical decompression | • 50–95% clot removed in 82% of patients |
| • >95% clot removed in 3 patients | |||
| • <50% clot removed in 1 patient | |||
| • Average thrombolysis time of 91 minutes | |||
| Elman et al. (16) | 2006 | Mechanical thrombectomy versus CDTL | • >50% clot removed in 93% of patients receiving mechanical thrombectomy |
| • >50% clot removed in 79% of patients receiving CDTL | |||
| Zurkiya et al. (10) | 2018 | CDTL with or without post surgical decompression balloon angioplasty | • 86% of patients undergoing CDTL had residual SV stenosis managed with balloon angioplasty |
| • 4 patients undergoing CDTL had chronic SV occlusion | |||
| Wooster et al. (8) | 2019 | Endovascular intervention with or without surgical decompression | • 67% of patients underwent endovascular repair including |
| • 23 PTA | |||
| • 13 Stent | |||
| • 18 venous reconstruction | |||
| Bashir et al. | 2022 | CDTL with surgical decompression | • Median thrombolysis time of 22 hours (6–46) |
| • 88% of patients had fully patent SV following decompression | |||
| • 100% of patients asymptomatic at follow-up |
CDTL, catheter-directed thrombolysis; PTA, percutaneous transluminal angioplasty; SV, subclavian vein.
The extent to which the residual SV stenosis experienced by the patients in the included series can be attributed to CDTL being performed over other thrombolysis methods is unclear. Data from Wooster suggests that endovascular intervention improves overall clinical outcome in patients with UEDVT (8). Sixty-seven percent of patients in their cohort underwent endovascular intervention, which included procedures such as PTA and even stent insertion. A 100% surgical success rate with symptomatic relief was observed in those undergoing endovascular intervention with surgical decompression. Furthermore, this approach was associated with low rates of SV reocclusion and symptom recurrence (9.4% and 11.3% respectively) (8). The clinical outcomes from the available literature scrutinising the use of CDTL and mechanical thrombectomy for PSS is summarised in Table 3. Others have described venous reconstruction for SV stenosis or occlusion and even homograft replacement. Molina describes an aggressive approach to SV stenosis with long term patency rates of over 90% following venous reconstruction (22-24). This means access to the SV is required posterior to the manubrium and may mean a proximal venous cross clamp is placed from the IC incision when a PC approach is planned. More proximal access may mean the manubrium needs to be split to gain safe access.
Other factors contributing to SV re-thrombosis are incomplete resection of the CCL and/or tendon of the subclavius muscle. The CCL is a rigid structure medial to the SV which can be visualised with both the TA and PC routes. It may also cause SV compression when its insertion into the clavicle is more lateral than expected (Figure 2). Similarly, the anteriorly lying subclavius muscle may be a further source of external compression on the SV and is easily identified by the IC route requiring its excision to enable exposure. Recent series comparing the SC to the IC route for SV decompression have shown improved patency rates with fewer post-operative symptoms with the IC approach (25). The surgical approach used in this current series was dependent on the surgeon with one favouring the TA and the other PC or IC approach.
The importance of carrying out FRR in conjunction with an endovascular intervention is clear—as emphasised by Zurkiya et al. (10): the degree of SV stenosis immediately post FRR is usually comparable to that of pre-decompression because anatomical resection does not deal with intrinsic wall lesions of the SV. This suggests prolonging the interval between thrombolysis and FRR, and indeed between FRR and any adjunctive intervention, could prove detrimental to clinical outcomes. The time from presentation to decompression >14 days is a documented factor associated with worse clinical outcome, as it may lead to early re-thrombosis warranting re-intervention (26).
Table 3: clinical outcomes
There is little current data concerning the open operative approach surgeons use to manage PSS. A survey of 60 United Kingdom (UK) members of the Vascular Surgical Society showed 4% performed thoracic outlet surgery for PSS by a PC incision with 55% using a TA and 28% the SC approach (27). Hence, at least in the UK, there appears to be considerable variation in surgical approaches used. This report dates back to 2004 and clinical practice has almost certainly significantly changed. Recent clinical practice guidelines on the management of venous thrombosis confirms timing of FRR for PSS remains controversial (28). A delayed approach to intervention may avoid unnecessary surgery if patients remain asymptomatic but which group this might apply to remains unknown.
Perhaps due to the low incidence of PSS, it may not be feasible for a sufficiently powered prospective randomised trial to be performed to gain a definitive answer as to the optimum surgical approach for SV decompression—particularly if the TA and PC/IC outcome differences are minimal. What seems increasing clear, however, are the added benefits of undertaking endovascular intervention alongside surgical decompression, both in terms of preoperative thrombolysis and postoperative adjunctive SV intervention. A recent meta-analysis and systematic review demonstrated higher rates of clot lysis and symptom improvement when accompanied by FRR for UEDVT (29). However, many of the studies included had small numbers or minimal data concerning any adjunctive techniques used. The conclusion was these limitations meant direct comparison between treatment arms was difficult to interpret.
A study examining pre-surgical decompression mechanical thrombectomy and/or CDTL might be appropriate. This study may aid management of PSS, and indeed UEDVT in general, and assess whether any benefit from treatment strategies traditionally employed to treat LEDVT would be seen. Modest improvements in SV patency are only clinically relevant if it can be shown to reduce the incidence of post thrombotic syndrome of the affected arm in the long term. So far, no significant association has been found with mechanical venous thrombectomy and needs further study. However, the long-term benefits can only be assessed if the incidence of post thrombotic syndrome decreases when treating those with PSS.
Recent advances in the management of PSS have included a robotic transthoracic approach which allows minimally invasive FRR and also reducing neurovascular complications (30). Also, the overall shift towards endovascular approaches has gained great popularity amongst surgeons managing complex cardiac and aortic pathologies—one needs only to look towards the advent of thoracic endovascular aortic repair as an example of the potential for endovascular procedures to successfully resolve complex cardiovascular surgical pathologies, without the need for large incisions and surgical trauma (30).
Conclusions
Despite the distinct lack of prospective, large-scale multi-centre data on the optimal management of PSS, existing literature indicates that a hybrid approach encompassing endovascular intervention combined with FRR yields respectable results that can be further improved by post-decompression venoplasty. Methods such as mechanical thrombectomy coupled with traditional CDTL are particularly promising potential approaches and established thromboembolic complications of PSS could potentially be avoided with the fitting of SVC filters. Excellent long-term SV patency rates are observed with both TA and PC/IC decompression approaches in the patients included in our series. Both approaches allow excellent access for complete resection of the anterior first rib, subclavius muscle and CCL eliminating any extrinsic compression precipitating the initial thrombotic event. Overall secondary patency rate was 88% with 100% asymptomatic. Currently, evidence is insufficient as to which patients may benefit from venous reconstruction but the IC approach enables this to be performed as the medial access to the SV is excellent.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Cardiovascular Diagnosis and Therapy for the series “Frozen Elephant Trunk”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-158/rc
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-158/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-158/coif). The series “Frozen Elephant Trunk” was commissioned by the editorial office without any funding or sponsorship. MB served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Urschel HC, Kourlis H. Thoracic outlet syndrome: a 50-year experience at Baylor University Medical Center. Proc (Bayl Univ Med Cent) 2007;20:125-35. [Crossref] [PubMed]
- Illig KA, Doyle AJ. A comprehensive review of Paget-Schroetter syndrome. J Vasc Surg 2010;51:1538-47. [Crossref] [PubMed]
- Schneider DB, Dimuzio PJ, Martin ND, et al. Combination treatment of venous thoracic outlet syndrome: open surgical decompression and intraoperative angioplasty. J Vasc Surg 2004;40:599-603. [Crossref] [PubMed]
- Vazquez FJ, Paulin P, Poodts D, et al. Preferred Management of Primary Deep Arm Vein Thrombosis. Eur J Vasc Endovasc Surg 2017;53:744-51. [Crossref] [PubMed]
- Landry GJ, Liem TK. Endovascular management of Paget-Schroetter syndrome. Vascular 2007;15:290-6. [Crossref] [PubMed]
- Lugo J, Tanious A, Armstrong P, et al. Acute Paget-Schroetter syndrome: does the first rib routinely need to be removed after thrombolysis? Ann Vasc Surg 2015;29:1073-7. [Crossref] [PubMed]
- Rigberg DA, Freischlag JA, Machleder HI. Vascular compression syndromes. In: Creager MA, Dzau V, Loscalzo J. editors. Vascular medicine: a companion to Braunwald’s heart disease. 1st edition. Philadelphia, PA, USA: Elsevier, 2006:920-33.
- Wooster M, Fernandez B, Summers KL, et al. Surgical and endovascular central venous reconstruction combined with thoracic outlet decompression in highly symptomatic patients. J Vasc Surg Venous Lymphat Disord 2019;7:106-12.e3. [Crossref] [PubMed]
- ATKINS HJ. Sympathectomy by the axillary approach. Lancet 1954;266:538-9. [PubMed]
- Zurkiya O, Donahue DM, Walker TG, et al. Safety and Efficacy of Catheter-Directed Therapies as a Supplement to Surgical Decompression in Venous Thoracic Outlet Syndrome. AJR Am J Roentgenol 2018;210:W80-5. [Crossref] [PubMed]
- Koury JP, Burke CT. Endovascular management of acute upper extremity deep venous thrombosis and the use of superior vena cava filters. Semin Intervent Radiol 2011;28:3-9. [Crossref] [PubMed]
- Lee MS, Singh V, Wilentz JR, et al. AngioJet thrombectomy. J Invasive Cardiol 2004;16:587-91. [PubMed]
- Villalba L, Nguyen T, Feitosa RL Jr, et al. Single-session catheter-directed lysis using adjunctive power-pulse spray with AngioJet for the treatment of acute massive and submassive pulmonary embolism. J Vasc Surg 2019;70:1920-6. [Crossref] [PubMed]
- Schneider DB, Curry TK, Eichler CM, et al. Percutaneous mechanical thrombectomy for the management of venous thoracic outlet syndrome. J Endovasc Ther 2003;10:336-40. [Crossref] [PubMed]
- Stewman C, Harwood M. Thoracic outlet syndrome. BMJ Best Practice 2020. Available online: https://bestpractice.bmj.com/topics/en-gb/592
- Elman EE, Kahn SR. The post-thrombotic syndrome after upper extremity deep venous thrombosis in adults: a systematic review. Thromb Res 2006;117:609-14. [Crossref] [PubMed]
- Likes K, Rochlin DH, Call D, et al. McCleery syndrome: etiology and outcome. Vasc Endovascular Surg 2014;48:106-10. [Crossref] [PubMed]
- Sanders RJ, Hammond SL. Subclavian vein obstruction without thrombosis. J Vasc Surg 2005;41:285-90. [Crossref] [PubMed]
- Shah AD, Bajakian DR, Olin JW, et al. Power-pulse spray thrombectomy for treatment of Paget-Schroetter syndrome. AJR Am J Roentgenol 2007;188:1215-7. [Crossref] [PubMed]
- Hilleman DE, Razavi MK. Clinical and economic evaluation of the Trellis-8 infusion catheter for deep vein thrombosis. J Vasc Interv Radiol 2008;19:377-83. [Crossref] [PubMed]
- O’Sullivan GJ, Lohan DG, Gough N, et al. Pharmacomechanical thrombectomy of acute deep vein thrombosis with the Trellis-8 isolated thrombolysis catheter. J Vasc Interv Radiol 2007;18:715-24. [Crossref] [PubMed]
- Molina JE. Reoperations after failed transaxillary first rib resection to treat Paget-Schroetter syndrome patients. Ann Thorac Surg 2011;91:1717-21. [Crossref] [PubMed]
- Molina JE, Hunter DW, Dietz CA. Protocols for Paget-Schroetter syndrome and late treatment of chronic subclavian vein obstruction. Ann Thorac Surg 2009;87:416-22. [Crossref] [PubMed]
- Molina JE. A new surgical approach to the innominate and subclavian vein. J Vasc Surg 1998;27:576-81. [Crossref] [PubMed]
- Bozzay JD, Walker PF, Ronaldi AE, et al. Infraclavicular Thoracic Outlet Decompression Compared to Supraclavicular Thoracic Outlet Decompression for the Management of Venous Thoracic Outlet Syndrome. Ann Vasc Surg 2020;65:90-9. [Crossref] [PubMed]
- Samoila G, Twine CP, Williams IM. The infraclavicular approach for Paget-Schroetter syndrome. Ann R Coll Surg Engl 2018;100:83-91. [Crossref] [PubMed]
- Khan SN, Stansby G. Current management of Paget-Schroetter syndrome in the UK. Ann R Coll Surg Engl 2004;86:29-34. [Crossref] [PubMed]
- Kakkos SK, Gohel M, Baekgaard N, et al. Editor’s Choice – European Society for Vascular Surgery (ESVS) 2021 Clinical Practice Guidelines on the Management of Venous Thrombosis. Eur J Vasc Endovasc Surg 2021;61:9-82. [Crossref] [PubMed]
- Karaolanis G, Antonopoulos CN, Koutsias SG, et al. A systematic review and meta-analysis for the management of Paget-Schroetter syndrome. J Vasc Surg Venous Lymphat Disord 2021;9:801-10.e5. [Crossref] [PubMed]
- Gharagozloo F, Meyer M, Tempesta B, et al. Robotic transthoracic first-rib resection for Paget-Schroetter syndrome. Eur J Cardiothorac Surg 2019;55:434-9. [Crossref] [PubMed]






