Low gradient “severe” aortic stenosis with preserved left ventricular ejection fraction
Introduction
In developed nations, aortic stenosis (AS) is the most common valvular heart disease presentation, and its prevalence is increasing due to aging populations. Our understanding of calcific AS has changed dramatically, from a concept of a "mechanical" disease of aging strictly limited with the valve, to a modern concept of a progressive disease process, with similarities to atherosclerosis. Because AS is a fatal disease when left untreated, replacement of the degenerative and stenotic valve with an artificial valve (bio-prosthetic or mechanical) is the recommended treatment for patients with symptomatic, severe AS. Therefore, the accurate diagnosis of the disease and determination of its hemodynamic severity are clinically essential in clinical decision-making. Current ACC/AHA guidelines recommend cut-off values for Doppler-echocardiographic measurements of severe aortic stenosis (AS) as follows: aortic valve area (AVA) <1.0 cm2 and/or indexed for body surface area <0.6 cm2/m2, mean gradient >40 mmHg and peak velocity >4.0 m/s (1). Similarly, ESC guidelines set the mean transvalvular gradient of >50 mmHg (2) (Table 1). However, inconsistent grading of AS remains a common problem in clinical practice, both in patients with impaired and normal LV functions. A common pattern is the combination of an AVA <1 cm2 with a gradient <40 mmHg.
Full Table
This pattern of “low-flow/low-gradient aortic stenosis” is relatively well known and accepted in AS patients with depressed LV function, where it was assumed that the failing LV cannot generate a high-flow/high-gradient across the stenotic valve. However, more recent observations demonstrate that “low-flow/low-gradient aortic stenosis” is not infrequently encountered in the setting of preserved LV EF (LV EF >50%). This clinical entity was therefore named “Paradoxical low flow and/or low gradient severe AS”. In a study of 2427 patients with aortic stenosis and preserved left ventricular systolic function evaluated at a referral centre, such discrepancies in grading were found in a surprisingly high percentage of almost one third of patients (30%). In these patients, valve area calculated by the continuity equation to less than 1 cm2, but the mean gradient was less than 40 mmHg (3). A subgroup of this cohort with inconsistent grading of AS interestingly represents with low flow across the valve, despite maintained LV function. Recent studies suggest that such patients are characterized by severe LV concentric hypertrophy with small LV cavity, restrictive filling pattern, and subtle myocardial dysfunction. Data suggest that these patients are at a more advanced stage of the disease process and have a poorer prognosis, if treated medically rather than surgically (4,5). This creates a challenging scenario for the clinicians by raising uncertainty regarding the actual severity and complicating an appropriate timely decision for AVR.
This review article, will discuss the reasons of inconsistent grading of aortic valve stenosis and possible pathophysiological mechanisms underlying low flow/low gradient severe AS, despite preserved LV EF, based on an illustrative case presentation.
Illustrative case
A 72-year-old woman with a history of hypertension, dyslipidemia and coronary artery disease presented with exertional dyspnea and angina. Four years ago, she was diagnosed with moderate aortic stenosis (Max/Mean Gradient 42/21 mmHg with a valve area 1.52 cm2) and coronary artery disease. At that time a lesion in the LAD was stented successfully.
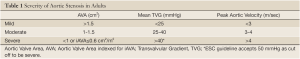
Transthoracic echocardiography was repeated to evaluate the current status of the patient. Severely calcified aortic stenosis was observed by 2D echo with transvalvular gradients measured at 53/32 mmHg and calculated AVA of 0.7 cm2 by continuity equation (Figure 1). There was normal LV systolic function with an ejection fraction (LV EF) of 64% by the biplane Simpson method. There was grade 2 diastolic dysfunction (Pseudo normal pattern with an E/E' ratio: 22) detected by a dedicated Doppler diastolic examination. Her blood pressure was 160/90 mmHg at the time of echo exam and her body surface area was 1.7 cm2. Coronary angiography and invasive left heart catheterization were performed to evaluate current coronary status and to determine AS severity (Figure 1). There was no severe coronary lesion for revascularization and LAD stent was patent.
How should this case be further evaluated?
Prevalence and clinical importance of low flow/low gradient severe AS with preserved LV EF
Hachicha et al. studied the clinical and Doppler echocardiographic data of 512 consecutive patients with severe aortic stenosis (iAVA ≤0.6 cm2/m2) and preserved LV EF (>50%) (6). Of these patients, 331 (65%) had normal LV flow output defined as a stroke volume index >35 mL/m2, and 181 (35%) had paradoxically low-flow output defined as stroke volume index ≤35 mL/m2. When compared with normal flow patients, low-flow patients had a higher prevalence of female gender (P<0.05), a lower transvalvular gradient (32±17 versus 40±15 mmHg; P<0.001), a lower LV diastolic volume index (52±12 versus 59±13 mL/m2; P<0.001), lower LV ejection fraction (62±8% versus 68±7%; P<0.001), a higher level of LV global afterload reflected by a higher valvulo-arterial impedance (5.3±1.3 versus 4.1±0.7 mm Hg·mL-1·m-2; P<0.001). Importantly, compared with normal flow patients, low-flow patients had a lower overall 3-year survival (76% versus 86%; P<0.006). The authors concluded that a subgroup of patients with severe aortic stenosis presents with low transvalvular flow and low gradients despite normal LV ejection fraction. Further, while the results of grading may lead to an undervaluation of severity with inappropriate delay of AVR, this pattern of the disease is in fact consistent with a more advanced stage of the disease and has a poorer prognosis (5). However, this study was a retrospective analysis and more importantly the symptomatic status of the included patients and details of the decision process leading to possible AVR were not comprehensively discussed.
Confirming prior results, data from Barasch et al. also demonstrated the impact of low gradient AS on outcome, reporting a two-fold increase in mortality and an almost 50% lower referral rate for AVR in the low-gradient AS compared to the high gradient group (4). In another report of 150 patients with severe AS and a normal LVEF, 12 patients with an invasively measured peak-to-peak gradient <50 mmHg were identified. Despite symptomatic status, AVR was not recommended. During 2.5 years of follow up, six patients had died (50% mortality) and three developed severe heart failure within (6). Contrary to these studies, Jander et al. found that patients with low-gradient “severe” aortic stenosis and normal ejection fraction have an outcome similar to that in patients with moderate aortic stenosis (7). The discordant results between these studies likely derive from differences in patient population and symptomatic status. Specifically, Jander et al. included asymptomatic AS patients from SEAS study (CAD, PAD, and DM were exclusion criteria), whereas 51 of the prospectively enrolled patients in the study by Barasch et al. were symptomatic. In a prospective trial, Rosenheck et al. demonstrated that outcomes of asymptomatic severe AS patients were almost the same with normal control in terms of event free survival (8).
Pathophysiology of low gradient severe AS in patients with preserved ejection fraction
Is the problem systolic dysfunction, although LV EF apparently appears normal?
In patient with AS, assessment of LV function plays a central role in the decision to proceed with AVR, and patients with preserved systolic function have an excellent outcome after AVR (9). However, it is well known that LV EF is a limited marker of systolic function, in particular in the setting of co-morbidities that affect LV mechanics (10,11). Calcific AS should not be considered as an isolated disease limited to the valve, but rather a manifestation of a more systemic disease process (12). Hypertension (13), the metabolic syndrome (14), atherosclerosis and aging are highly prevalent in AS patients. These conditions cause direct alterations of LV mechanics and also contribute to vascular stiffness and increased afterload.
Briand et al. suggested that increased afterload should be taken into account when assessing AS severity (15). The authors demonstrate that valvuloarterial impedance (Zva), which is calculated by dividing the total systolic pressure (systolic blood pressure plus mean transvalvular gradient) by the stroke volume index (SVi) reflects increased afterload in AS patients. Zva, in fact, represents the cost in mmHg for each systemic millimeter of blood indexed for the body size. The data demonstrates that increased afterload is strongly associated with LV systolic and diastolic dysfunction in AS patients. Hachica et al. further demonstrated that increased Zva (>3.5 mmHg·mL-1·m2) identifies poor outcome in asymptomatic severe AS patients (16). Low gradient severe AS with preserved LV EF patients were also evaluated in a SEAS sub study and interestingly Zva was found as the main determinant of LV dysfunction in asymptomatic patients (17). These data suggest that measures of afterload, including an increased Zva can guide risk stratification and follow up decisions and are incremental to measures of LVEF.
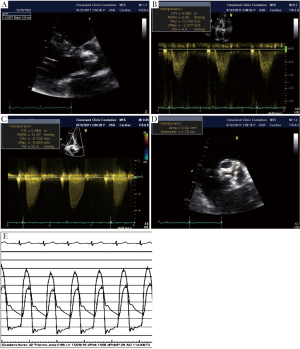
The development of myocardial fibrosis, independent of prior myocardial infarction, has been well established in severe AS patients and a direct relationship has been shown between severity of fibrosis and adverse outcome (18). In a recent study with severe symptomatic AS patients, low gradient was found to be associated with higher degree of fibrosis and decreased longitudinal function and poorer clinical outcome despite preserved LV EF (19). Mitral ring displacement was shown to differentiate between moderate AS and low gradient severe AS with preserved EF. Subtle myocardial dysfunction and decreased longitudinal deformation have been demonstrated in studies using different techniques such as M mode echocardiography (20), tissue Doppler imaging (TDI) (21) and 2D Speckle Tracking Echocardiography (2D STE) (22,23) (Figure 2). In patients with low-flow/low-gradient AS despite preserved EF, decreased global longitudinal strain was found and it was inversely related with increased global afterload as calculated by Zva (24).
Is the problem “simply” LV diastolic dysfunction?
Diastolic dysfunction in varying degrees is highly prevalent in aging populations due to associated co-morbid factors such as coronary artery disease, hypertension, and diabetes mellitus. Both impaired myocardial relaxation and increased stiffness are contributing to impaired LV diastolic filling. Increased filling pressure with severe LV diastolic dysfunction has been associated with worse outcome in AS patients. A restrictive filling pattern with a small LV cavity has been found to be more common in LGAS patient (5). However, each factor including atrial fibrillation, mitral annular calcification, severe pulmonary hypertension etc. can affect LV filling negatively and this may results with low cardiac output.
Methodological explanations for inconsistent grading of severity of aortic stenosis
Definition of cut off values
Current guidelines state that “when AS is severe and cardiac output is normal, the mean transvalvular pressure gradient is generally >40 mmHg”. However as described above, discrepancy between the valve area (<1 cm2) and transvalvular gradients (<40 mmHg) are frequent in both invasive measurements during catheterization and non invasive calculation by echocardiography.
Some of this inconsistency may be related matching of the cut-off values, as has been suggested based on invasive measurements. The Gorlin formula calculates AVA as:
Carabello demonstrated the potential mismatch of the cutoff values with the following assumption (25). A cardiac output of 6 L/min, systolic ejection period of 0.33 seconds, and heart rate of 80 beats per minute, a mean gradient of 26 mmHg actually yields to an AVA of 1.0 cm2, whereas a mean gradient >40 is corresponding with a AVA of 0.8 cm2.
These values are similar to data observed in the above cited study of 2427 patients with aortic stenosis and preserved left ventricular systolic function (3). In this study a substantial proportion of patients (30%) had a valve area calculated by the continuity equation less than 1 cm2, but a mean gradient also less than 40 mmHg (3). Similar to the results by Carabello, an AVA of 1 cm2 was found to be correlated with a mean gradient of 22.8 mmHg. Conversely, a mean gradient of 40 mmHg was found to be correlated with an in vivo AVA of 0.75 cm2 and maximum velocity of 4 cm/sec was corresponding with an in vivo AVA of 0.82 cm2.
These data suggest that the echocardiographic and invasive results for AVA and trans-valvular gradient are actually consistent, but that the proposed cut-off values have limitations.
Small body size
AVA indexed for the body size is an important step in the assessment of discrepant measurement. In current guidelines, an indexed AVA cut off value of 0.6 cm2/m2 is recommended as the criterion for severe AS (1,2). Patients with small body surface area can be incorrectly classified as having severe AS, if the AVA is not indexed. Conversely, a large body size may cause an overestimation of AVA in terms of disease severity unless it is indexed for BSA. For example, an AVA of 0.9 cm2 (iAVA= 0.7 cm2/m2) does not indicate severe AS in a woman with a BSA of 1.3 m2, whereas an AVA of 1.2 cm2 (iAVA=0.57 cm2/m2) should be considered as severe in a man with a BSA of 2.1 m2.
Measurement error and possible pitfalls of LVOT measurements
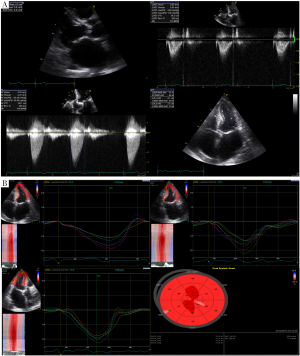
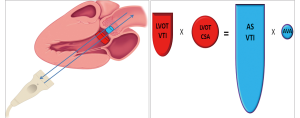
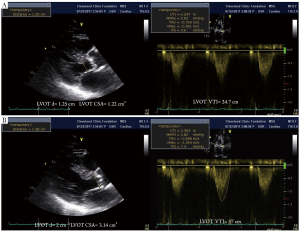
It is important to note that LVOT measurements play an essential role for several reasons. First, calculation of AVA by continuity equation (Figure 3) depends on the area of the LVOT measurement (26). Second, calculation of stroke volume is derived from pulsed wave Doppler of LVOT. Third, estimation of global load by using valvuloarterial impedance (Zva) requires accurate LVOT measurements. All these parameters change substantially with variations in LVOT measurements. Therefore, if there occurs any mistake in LVOT measurements, AVA, SV and Zva will be calculated wrong. Figure 4 depicts the importance of LVOT measurements.
Contamination of the LVOT with mitral regurgitation signal and erroneous correction for misalignment of Doppler beam can be the other potential pitfalls.
How should we approach a patient with low gradient and severe AS?
The accurate assessment of the hemodynamic severity of AS is clinically essential for several reasons. First, determination of the severity of the disease is critical to explain the symptomatic status. If the AS is not severe, differential diagnosis should be carefully reviewed and possible other reasons such as coronary artery disease or hypertensive heart disease should be ruled out. Second, since AS is a progressive disease, quantitative measures allow monitoring and predicting disease progression for optimal timing of valve replacement. Third, if the patient is totally asymptomatic, evaluation of disease severity provides critical data in terms of risk stratification and future therapeutic decision.
In the setting of inconsistently grading AS despite preserved LV EF, possible measurement pitfalls should be reviewed and the symptomatic status of the patients should be clarified. If the patient is symptomatic with an indexed AVA of <0.6 cm2/m2, AVR should be recommended. If symptoms are equivocal, measures of afterload and in particular Zva can be calculated in order to estimate total load. In addition measuring global LV strain can provide important data in terms of LV function and risk stratification. However, it is noteworthy that neither Zva nor global longitudinal strain can differentiate moderate and severe AS. Zva reflects increased global load but unfortunately cannot discriminate the load separately. Dobutamine stress echo can be carefully performed in order to assess contractile reserve. B type Natriuretic Peptide (BNP) can provide useful data regarding risk assessment, and other imaging modalities such as TEE and 3D TEE can better characterize LVOT measurement and finally calcium score of aortic valve can give us an idea about disease severity.
Since there is no prospective randomized trial about outcome of low-flow/low-gradient patients despite preserved LV to date, utility of aforementioned parameters (Zva, global longitudinal strain, contractile reserve with DSE or calcium score of the valve) is incompletely understood and clinical decision should be tailored individually.
Commentary on illustrative case
In our illustrative case the patient was symptomatic and had severe AS. Invasive study confirmed that this patient had “normal flow/low gradient and severe AS despite preserved LV EF”. SVi was calculated as 42 mL/m2 and Zva was calculated as 4.5 mmHg·mL-1·m-2. This case is a good example of inconsistently grading AS.
Conclusions
Inconsistent results of grading AS remains a common problem in the assessment of patient with suspected severe aortic stenosis. An important subgroup of these patients has ‘paradoxically' low-flow/low-gradient severe AS despite preserved LV EF. This pattern of the AS is consistent with a more advanced stage of the disease and has a poorer prognosis. The inconsistencies in grading may leads to an undervaluation of symptoms and inappropriate delay of AVR. Although accurate assessment of the hemodynamic severity of AS is clinically essential for decision making, symptomatic status of this cohort should be carefully evaluated and clinical decision should be tailored individually.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- American College of Cardiology/American Heart Association Task Force on Practice Guidelines, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation 2006;114:e84-231.
- Vahanian A, Baumgartner H, Bax J, et al. Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J 2007;28:230-68.
- Minners J, Allgeier M, Gohlke-Baerwolf C, et al. Inconsistencies of echocardiographic criteria for the grading of aortic valve stenosis. Eur Heart J 2008;29:1043-8.
- Barasch E, Fan D, Chukwu EO, et al. Severe isolated aortic stenosis with normal left ventricular systolic function and low transvalvular gradients: pathophysiologic and prognostic insights. J Heart Valve Dis 2008;17:81-8.
- Hachicha Z, Dumesnil JG, Bogaty P, et al. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation 2007;115:2856-64.
- Christensen KL, Ivarsen HR, Thuesen L, et al. Aortic valve stenosis: fatal natural history despite normal left ventricular function and low invasive peak-to-peak pressure gradients. Cardiology 2004;102:147-51.
- Jander N, Minners J, Holme I, et al. Outcome of patients with low-gradient "severe" aortic stenosis and preserved ejection fraction. Circulation 2011;123:887-95.
- Rosenhek R, Binder T, Porenta G, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med 2000;343:611-7.
- Lindblom D, Lindblom U, Qvist J, et al. Long-term relative survival rates after heart valve replacement. J Am Coll Cardiol 1990;15:566-73.
- Aurigemma GP, Silver KH, Priest MA, et al. Geometric changes allow normal ejection fraction despite depressed myocardial shortening in hypertensive left ventricular hypertrophy. J Am Coll Cardiol 1995;26:195-202.
- Ballo P, Mondillo S, Motto A, et al. Left ventricular midwall mechanics in subjects with aortic stenosis and normal systolic chamber function. J Heart Valve Dis 2006;15:639-50.
- Pibarot P, Dumesnil JG. Aortic stenosis: look globally, think globally. JACC Cardiovasc Imaging 2009;2:400-3.
- Linhartová K, Filipovský J, Cerbák R, et al. Severe aortic stenosis and its association with hypertension: analysis of clinical and echocardiographic parameters. Blood Press 2007;16:122-8.
- Pagé A, Dumesnil JG, Clavel MA, et al. Metabolic syndrome is associated with more pronounced impairment of left ventricle geometry and function in patients with calcific aortic stenosis: a substudy of the ASTRONOMER (Aortic Stenosis Progression Observation Measuring Effects of Rosuvastatin). J Am Coll Cardiol 2010;55:1867-74.
- Briand M, Dumesnil JG, Kadem L, et al. Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: implications for diagnosis and treatment. J Am Coll Cardiol 2005;46:291-8.
- Hachicha Z, Dumesnil JG, Pibarot P. Usefulness of the valvuloarterial impedance to predict adverse outcome in asymptomatic aortic stenosis. J Am Coll Cardiol 2009;54:1003-11.
- Cramariuc D, Cioffi G, Rieck AE, et al. Low-flow aortic stenosis in asymptomatic patients: valvular-arterial impedance and systolic function from the SEAS Substudy. JACC Cardiovasc Imaging 2009;2:390-9.
- Weidemann F, Herrmann S, Störk S, et al. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation 2009;120:577-84.
- Herrmann S, Störk S, Niemann M, et al. Low-gradient aortic valve stenosis myocardial fibrosis and its influence on function and outcome. J Am Coll Cardiol 2011;58:402-12.
- Takeda S, Rimington H, Smeeton N, et al. Long axis excursion in aortic stenosis. Heart 2001;86:52-6.
- Giorgi D, Di Bello V, Talini E, et al. Myocardial function in severe aortic stenosis before and after aortic valve replacement: a Doppler tissue imaging study. J Am Soc Echocardiogr 2005;18:8-14.
- Delgado V, Tops LF, van Bommel RJ, et al. Strain analysis in patients with severe aortic stenosis and preserved left ventricular ejection fraction undergoing surgical valve replacement. Eur Heart J 2009;30:3037-47.
- Ozkan A, Kapadia S, Tuzcu M, et al. Assessment of left ventricular function in aortic stenosis. Nat Rev Cardiol 2011;8:494-501
- Lee SP, Kim YJ, Kim JH, et al. Deterioration of myocardial function in paradoxical low-flow severe aortic stenosis: two-dimensional strain analysis. J Am Soc Echocardiogr 2011;24:976-83.
- Carabello BA. Clinical practice. Aortic stenosis. N Engl J Med 2002;346:677-82.
- O'Brien B, Schoenhagen P, Kapadia SR, et al. Integration of 3D imaging data in the assessment of aortic stenosis: impact on classification of disease severity. Circ Cardiovasc Imaging 2011;4:566-73.


