Outcomes in hypertrophic cardiomyopathy patients with and without atrial fibrillation: a survival meta-analysis
Introduction
Hypertrophic cardiomyopathy (HCM) is the most commonly inherited cardiomyopathy (1). It is associated with sudden cardiac death (SCD) and progressive ventricular dysfunction (2,3). It is a complex syndrome with variable cardiac pathology including atrial and ventricular arrhythmias, systolic and diastolic dysfunction, mitral valve disorders, left ventricular (LV) hypertrophy, left ventricular outflow tract (LVOT) obstruction (4,5). Atrial fibrillation (AF) is a common occurrence in patients with HCM, with a recently published meta-analysis of 33 studies reporting a prevalence of 22.45% (6). AF is associated with adverse outcomes in HCM patients with a varied phenotypic and clinical profile as compared to those in normal sinus rhythm (7-13). This is thought mainly to be related to stroke risk, worsening heart failure symptoms and poor functional capacity. However, there is a suggestion that there is an increased risk of SCD after excluding underlying stroke and heart failure related deaths (7,8), with the mechanism remaining largely unknown. Currently there are no prospective studies or randomized controlled trials in patients with HCM who have or develop AF. However, multiple observational studies examined the association between the development of AF in HCM patients and increased risk of death (7-14,15). Given the individual differences among these studies and the mixed nature of the HCM population evaluated, we performed a meta-analysis of all studies reporting on the mortality in HCM patients with and without AF to further understand the relationship between AF in HCM and mortality.
Methods
We conducted a meta-analysis of observational studies following MOOSE (Meta-analysis of Observational Studies in Epidemiology) guidelines (16).
Literature review
We searched five databases including PubMed, Medline, Embase, Ovid and Cochrane for relevant studies (Jan 1980–August 2015). This search was independently conducted by two investigators (A.M and M.Y.D). Key terms used were “atrial fibrillation in hypertrophic cardiomyopathy”, “atrial fibrillation outcomes hypertrophic cardiomyopathy”, and “hypertrophic cardiomyopathy outcome”. The search parameters were limited to English language only. Abstracts and full texts of potential studies were reviewed.
Study selection and inclusion criteria
Studies evaluating AF in HCM were reviewed. Studies were selected on the basis of reporting on HCM patients with and without AF, comparing mortality (cardiac mortality, all-cause mortality, or both) and outcomes in both groups.
Exclusion criteria
(I) Studies reporting on patients without HCM; (II) Absence of mortality as an outcome; (III) Duplicate studies either from the same author, same institution or similar patient population studied.
Data extraction
Relevant information was collected independently by two investigators (A.M and M.Y.D). This included (but not limited to): age, gender, comorbidities, symptoms, medications, family history of SCD, LVOT obstruction, left atrium volume index (LAVI), left atrial dimensions, LV thickness, LV ejection fraction (LVEF), % of patients in New York Heart Association (NYHA) class III/IV, presence of an internal cardiovertor-defibrillator (ICD), and percentage of patients undergoing invasive approach with either myectomy (+/− other surgeries) or alcohol septal ablation.
Outcome
Primary outcome was mortality across all studies over the follow up period (including aborted SCD if reported). Multiple secondary analyses were performed, including (I) cardiac mortality; (II) cardiac mortality related to heart failure or SCD; (III) cardiac mortality excluding stroke related mortality; (IV) meta-analysis of adjusted hazard ratios (HR) was reported when available
Statistical analysis
Continuous variables are expressed as mean and/or median and compared using Student’s t-test. Categorical data are expressed as percentage. We used Mantel Haenszel odds ratio (OR) utilizing DerSimonian and Laird’s random-effect models (17). When combing hazard ratios, study-specific estimates were combined using inverse variance-weighted averages of logarithmic HR in random-effects model. Statistical significance was set at a P value <0.05 (2-tailed). We assessed heterogeneity among studies using the inconsistency index (I2) statistic (range from 0% to 100%, I2>60% designate significant heterogeneity (18). Potential publication bias was evaluated by Begg’s funnel plot method (19). In order to estimate the total number of events in each group when not reported, annual rate of event was multiplied by the mean of follow up.
In order to further analyze our results and detect any potential heterogeneity contributed by an individual study, we performed one study removed meta-analysis; where each time the analysis is done by removing one study at a time and evaluating the change in the measured effect and the reported heterogeneity. All analyses performed using Review Manager (version 5.3, The Cochrane Collaboration, Oxford, USA) and SPSS version 11.5 (SPSS Inc., Chicago, IL, USA).
Results
Study selection
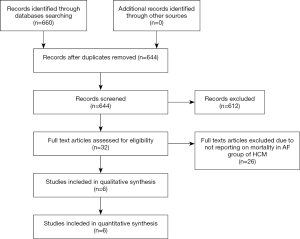
Initial search yielded 660 studies, after screening titles and/or abstracts, 32 full texts were reviewed. We included 6 studies (all observational studies; Figure 1). Search did not yield any randomized trials.
Characteristics of included studies
Baseline characteristics of individual studies are summarized in Table 1. There were 6,858 patients with history of HCM; 1,314 (19%) had coexistent AF either at baseline or developed during follow up. Mean follow up duration ranged between 4 and 8 years. Four studies directly evaluated the relationship between developing AF in HCM and outcomes (7,9,10,15). The other two studies looked at HCM epidemiology and reported mortality in patients with AF as compared to those without (8,14).
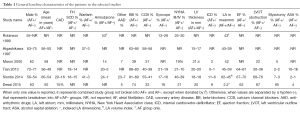
Full table
Outcomes
The individual study breakdown of number of events and mode of death in patients with HCM with or without AF are outlined in Table 2. Two studies (10,15) reported only all-cause mortality, while the other four studies reported on cardiac mortality (7-9,14).
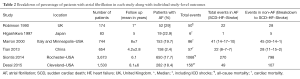
Full table
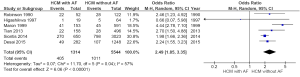
- Combining all studies (all-cause mortality and cardiac mortality): Out of 6,858 patients, 1,416 (20.6%) patients died. 405 or 30.8% of patients with AF died as compared to 1,011 or 18.2% of patients without AF (OR =2.49; 95% CI: 1.85–3.35, P<0.00001, I2=57%) (Figure 2). Funnel plot for the included studies is shown in Figure 3;
- Cardiac mortality (excluding studies reporting only all-cause mortality): Out of 3,011 patients, 298 (10%) died during follow up. 113 or 18% of patients with AF died from cardiac cause as compared to 185 or 8% of patients without AF (OR =2.8; 95% CI: 1.79–4.39, P<0.00001, I2=56%) (Figure 4). Funnel plot is shown in Figure 5. Cardiac mortality (excluding deaths related to stroke). Two studies reported on the specific mode of cardiac death (7,8). Out of 1398 patients, 116 (8.3%) patients died. 46 or 14% of patients with AF died from cardiac cause other than stroke-related deaths as compared to 70 (6.4%) patients without AF (OR =2.57; 95% CI: 1.57–4.20, P=0.0002, I2=31%) (Figure 6);
- Meta-analysis of adjusted hazard ratios predicting the outcome of mortality (all-cause/cardiac). Three studies (5,857 patients) reported on HR for the presence of AF in HCM patients as compared to absence of AF; the weighted HR was 1.66 (95% CI: 1.29–2.13, P<0.0001, I2=41%) (Figure 7);
- Sensitivity analysis. A one study removed Sensitivity analysis was performed (after removal of 1 study, Maron et al. (8) on the primary outcome (i.e., combined all-cause mortality and cardiac mortality among all 6 studies) to examine the contribution of each study and to examine sources of heterogeneity. Results of the estimated effect persisted with the removal of one study at a time across all analyses (six total analyses were performed). Exclusion of one study by Maron et al. (8) resulted in similar results but improved homogeneity and narrower CI (OR =2.07; 95% CI: 1.79–2.41, P<0.00001, I2=0%). Removal of the largest study by Siontis resulted in similar results (OR =2.76, 95% CI: 1.94–3.93, P≤0.00001, I2=42%).
Discussion
In this current systematic review and meta-analysis of the impact of development or presence of AF on outcomes in patients with HCM, we show that in a large cohort of patients with HCM, AF was common, with a prevalence of 19%, and was associated with more cardiac events/mortality as compared to HCM patients without AF. This finding persisted even after the inclusion of studies reporting on cardiac mortality only.
In terms of the magnitude of effect on mortality posed by the development of AF in HCM, few studies examined the presence of AF as a predictor of mortality. A recently published study by our group that is included in this meta-analysis (14), showed that AF is a predictor of cardiac mortality and ICD shocks in multivariate analysis with HR of 1.90 (95% CI: 1.32–2.72, P<0.001). Similarly, a study from Mayo Clinic reported that AF is a predictor of all-cause mortality with HR of 1.76 (95% CI: 1.51–2.03) (10), with the association persisted after adjusting for age, sex, family history of SCD, NYHA class III/IV, and obstructive phenotype. We attempted to do a meta-analysis of reported HR across studies. However, only 3 studies (5,857 patients) reported on HR for the presence of AF; weighted HR of 1.66 (95% CI: 1.29–2.13, P<0.0001, I2=41%).
AF in HCM is common, with a prior meta-analysis of 7,381 HCM patients showing an overall prevalence of 22.45% (6). Currently, there are no randomized trials in patients with HCM with or without AF. Prior meta-analyses published evaluated the prevalence/incidence of AF in HCM, LA size, and risk of thromboembolism in that group (6). However, since these analyses included a wide range of studies with broad selection criteria, no analysis on mortality outcome was reported. In this current analysis, our main goal was to analyze mortality among published studies, thus limiting the number of included studies but still with a large cohort of patients.
The optimal management strategy in HCM patients with concomitant AF is yet to be determined. Some studies looked at the use of antiarrhythmic (primarily Amiodarone) (15,20-22), while others evaluated more invasive strategies such as catheter based AF ablation or surgical based intervention (23-26). It remains poorly understood why HCM patients with AF undergoing ablation (either catheter based or surgical) have higher rates of recurrence as compared to AF in non-HCM patients (23-27). A recent met-analysis have shown how it is feasible to perform catheter ablation for HCM patients with AF, but with a 32.9% single procedure success without anti-arrhythmics, that rose to 50.4% with multiple procedures (28). Currently, there is not enough data evaluating incidence of cardiovascular events or mortality in patients with HCM and AF undergoing invasive interventions to eliminate AF as compared to those receiving medications only (for either rhythm or rate control). It would be prudent to evaluate these strategies in a prospective and/or randomized fashion to see if they have an effect on mortality or morbidity of HCM patients.
HCM is a complex syndrome, with risk of SCD through ventricular arrhythmias (1,3). AF initially was not thought to be associated of excess mortality in HCM patients (15). However, since then, many reports from various parts of the world have shown worse prognosis with the presence of AF even after adjusting for various risk factors (7-14). Mortality related to AF could be explained by various pathophysiological mechanisms that occur in patients with AF without underlying HCM, which is usually related to thromboembolism (stroke or systemic embolism), related to side effects of warranted anticoagulation, particularly intracranial bleed, or related to the development of systolic dysfunction and progressive heart failure (27). However, in HCM patients, some studies have shown AF to be associated with increased risk of SCD after excluding underlying stroke and heart failure related deaths (7,8), with the mechanism remaining largely unknown. In some reports, it was theorized that AF might trigger ventricular arrhythmias (29,30), reduce ventricular tissue refractory period (30), pro-arrhythmia through the short-long-short cycle length sequence (31) and it affects cardiac output through loss of atrial kick (32), Also, AF could be a sign of increased catecholamines that also would be an underlying stimulus for ventricular tachycardia (33). In the current analysis, only 2 studies in 1,398 patients (7,8) reported on the specific cause of cardiac death, and after excluding stroke-related deaths, AF was still associated with increased mortality (OR =2.57; 95% CI: 1.57–4.2, P=0.0002, I2=31%). However, from the perspective of ICD recommendations, based on current guidelines (1,3), presence of AF is not considered to be a major risk factor for SCD. However, based on results of the current meta-analysis and above reported data (11-13), it appears that there might be an association between AF and SCD. If so, then such patients (especially those with permanent AF) might need to be evaluated for an ICD. However, these findings require prospective validation.
Limitations
As with any meta-analysis of studies reporting on uncommon diseases, there was a lack of randomized trials. Even though HCM is relatively common, all published literature is observational in nature, but it represents the basis of most guidelines’ driven recommendations in HCM patients and current best practice. Hence, our aim was to evaluate the published literature and thus all included studies were observational in nature. Another limitation is that we included only six studies. This is related to having a strict inclusion criteria looking specifically at mortality in patients with HCM and AF as compared to HCM patients without AF. Also, we excluded studies reporting on the same patient population, or studies published from same hospital or the same research group. This led to us being very restrictive in terms of study inclusion since most published literature on HCM comes from few centers around the world. However, those six studies included a large number of patients to analyze, and with varied geographical distribution, making them a very good representative cohort. Given the observational nature of the included studies, data on the mode of death if it was sudden cardiac death secondary to ventricular arrhythmias were limited; thus we used cardiac and all-cause mortality in the primary outcome. Also, the use of ICDs was not universal across studies, so reporting on the benefit of ICDs in modulating the risk of mortality associated with AF in HCM population is not possible and beyond the scope of this manuscript. Finally, studies varied in the reporting on known co-existent risk factors for mortality or SCD in HCM patients. So there could be confounding in reporting on HCM patients with AF. However, the largest studies examined in a multivariate model the association between AF and mortality after adjusting for known risk factors and the association between AF and mortality remained significant (10,14).
Conclusions
Patients with HCM who develop AF have higher risk of all-cause mortality and cardiac deaths as compared to HCM patients without AF, with an increased incidence of sudden cardiac death, heart failure related deaths, and stroke related deaths. There is a need for a clinical trial comparing the current various treatment strategies for AF in HCM; to determine which strategy would best modulate the increased risk of death in this population.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011;124:e783-831. [Crossref] [PubMed]
- Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA 2002;287:1308-20. [Crossref] [PubMed]
- Authors/Task Force members, Elliott PM, Anastasakis A, Borger MA, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2733-79. [Crossref] [PubMed]
- Maron MS, Olivotto I, Betocchi S, et al. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med 2003;348:295-303. [Crossref] [PubMed]
- Elliott PM, Gimeno JR, Tomé MT, et al. Left ventricular outflow tract obstruction and sudden death risk in patients with hypertrophic cardiomyopathy. Eur Heart J 2006;27:1933-41. [Crossref] [PubMed]
- Guttmann OP, Rahman MS, O'Mahony C, et al. Atrial fibrillation and thromboembolism in patients with hypertrophic cardiomyopathy: systematic review. Heart 2014;100:465-72. [Crossref] [PubMed]
- Tian T, Wang Y, Sun K, et al. Clinical profile and prognostic significance of atrial fibrillation in hypertrophic cardiomyopathy. Cardiology 2013;126:258-64. [Crossref] [PubMed]
- Maron BJ, Olivotto I, Spirito P, et al. Epidemiology of hypertrophic cardiomyopathy-related death: revisited in a large non-referral-based patient population. Circulation 2000;102:858-64. [Crossref] [PubMed]
- Higashikawa M, Nakamura Y, Yoshida M, et al. Incidence of ischemic strokes in hypertrophic cardiomyopathy is markedly increased if complicated by atrial fibrillation. Jpn Circ J 1997;61:673-81. [Crossref] [PubMed]
- Siontis KC, Geske JB, Ong K, et al. Atrial fibrillation in hypertrophic cardiomyopathy: prevalence, clinical correlations, and mortality in a large high-risk population. J Am Heart Assoc 2014;3:e001002. [Crossref] [PubMed]
- Desai MY, Bhonsale A, Smedira NG, et al. Predictors of long-term outcomes in symptomatic hypertrophic obstructive cardiomyopathy patients undergoing surgical relief of left ventricular outflow tract obstruction. Circulation 2013;128:209-16. [Crossref] [PubMed]
- Desai MY, Bhonsale A, Patel P, et al. Exercise echocardiography in asymptomatic HCM: exercise capacity, and not LV outflow tract gradient predicts long-term outcomes. JACC Cardiovasc Imaging 2014;7:26-36. [Crossref] [PubMed]
- Masri A, Pierson LM, Smedira NG, et al. Predictors of long-term outcomes in patients with hypertrophic cardiomyopathy undergoing cardiopulmonary stress testing and echocardiography. Am Heart J 2015;169:684-692.e1. [Crossref] [PubMed]
- Desai MY, Smedira NG, Bhonsale A, et al. Symptom assessment and exercise impairment in surgical decision making in hypertrophic obstructive cardiomyopathy: Relationship to outcomes. J Thorac Cardiovasc Surg 2015;150:928-35.e1. [Crossref] [PubMed]
- Robinson K, Frenneaux MP, Stockins B, et al. Atrial fibrillation in hypertrophic cardiomyopathy: a longitudinal study. J Am Coll Cardiol 1990;15:1279-85. [Crossref] [PubMed]
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. [Crossref] [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [Crossref] [PubMed]
- Higgins JPT GSe. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Available online: www.cochrane-handbook.org
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. [Crossref] [PubMed]
- Maron BJ, Olivotto I, Bellone P, et al. Clinical profile of stroke in 900 patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 2002;39:301-7. [Crossref] [PubMed]
- Olivotto I, Cecchi F, Casey SA, et al. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation 2001;104:2517-24. [Crossref] [PubMed]
- Cecchi F, Olivotto I, Montereggi A, et al. Hypertrophic cardiomyopathy in Tuscany: clinical course and outcome in an unselected regional population. J Am Coll Cardiol 1995;26:1529-36. [Crossref] [PubMed]
- Di Donna P, Olivotto I, Delcrè SD, et al. Efficacy of catheter ablation for atrial fibrillation in hypertrophic cardiomyopathy: impact of age, atrial remodelling, and disease progression. Europace 2010;12:347-55. [Crossref] [PubMed]
- Bunch TJ, Munger TM, Friedman PA, et al. Substrate and procedural predictors of outcomes after catheter ablation for atrial fibrillation in patients with hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol 2008;19:1009-14. [Crossref] [PubMed]
- Kilicaslan F, Verma A, Saad E, et al. Efficacy of catheter ablation of atrial fibrillation in patients with hypertrophic obstructive cardiomyopathy. Heart Rhythm 2006;3:275-80. [Crossref] [PubMed]
- Bassiouny M, Lindsay BD, Lever H, et al. Outcomes of nonpharmacologic treatment of atrial fibrillation in patients with hypertrophic cardiomyopathy. Heart Rhythm 2015;12:1438-47. [Crossref] [PubMed]
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130:2071-104. [Crossref] [PubMed]
- Ha HS, Wang N, Wong S, et al. Catheter ablation for atrial fibrillation in hypertrophic cardiomyopathy patients: a systematic review. J Interv Card Electrophysiol 2015;44:161-70. [Crossref] [PubMed]
- Stafford WJ, Trohman RG, Bilsker M, et al. Cardiac arrest in an adolescent with atrial fibrillation and hypertrophic cardiomyopathy. J Am Coll Cardiol 1986;7:701-4. [Crossref] [PubMed]
- Boriani G, Rapezzi C, Biffi M, et al. Atrial fibrillation precipitating sustained ventricular tachycardia in hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol 2002;13:954. [Crossref] [PubMed]
- Denker S, Lehmann M, Mahmud R, et al. Facilitation of ventricular tachycardia induction with abrupt changes in ventricular cycle length. Am J Cardiol 1984;53:508-15. [Crossref] [PubMed]
- Lerman BB. Mechanoelectrical feedback: maturation of a concept. J Cardiovasc Electrophysiol 1996;7:17-9. [Crossref] [PubMed]
- Lown B, Verrier RL. Neural activity and ventricular fibrillation. N Engl J Med 1976;294:1165-70. [Crossref] [PubMed]