High residual platelet reactivity on clopidogrel: its significance and therapeutic challenges overcoming clopidogrel resistance
Dual antiplatelet therapy with aspirin and clopidogrel has been the cornerstone in the management of coronary artery disease in patients with Acute Coronary Syndrome (ACS) and those undergoing Percutaneous Coronary Intervention (PCI) (1-3) with clear benefits in the prevention of stent thrombosis following PCI (4). However, despite dual antiplatelet therapy, many patients continue to suffer recurrent ischemic events. This has been partly attributed to High residual platelet reactivity (HRPR) or high on-treatment platelet reactivity (HTPR). HRPR may be defined as the high level of platelet reactivity that is present hours after receiving a loading dose of an antiplatelet agent (5). In most prospective studies, resistance to clopidogrel has been the most extensively studied. The higher the residual platelet reactivity is the higher the risk of cardiovascular adverse events.
In this review we examine the clinical significance of HRPR or HTPR, the various tests commonly used to quantify it, the proposed strategies, as well as the novel therapeutic options for this clinical challenge.
Definining clopidogrel resistance
Whereas clopidogrel resistance has been viewed as the occurrence of an adverse cardiovascular event while a patient is on clopidogrel therapy, the most acceptable definition of resistance/nonresponsiveness to an antiplatelet agent is the failure of the drug to inhibit its target of action (6). The objective criterion to verify resistance should therefore be based on a laboratory technique that can quantify the activity of the target receptor both before and after administration of the antiplatelet drug: the residual platelet reactivity. The association between high on-treatment platelet reactivity (HTPR) and future (periprocedural and long-term) ischemic risk has been strongly suggested in the literature, notwithstanding the lack of an optimal method to define HTPR and risk stratify the patients. Furthermore, the exact timing of measurement of platelet reactivity is not established (6).
High Residual Platelet reactivity (HRPR) may be defined as the high level of platelet reactivity that is present hours after receiving a loading dose of an antiplatelet agent (5). In most prospective studies, clopidogrel has been most extensively studied; the higher the residual platelet reactivity the higher the risk of cardiovascular adverse events.
Available platelet functional assays
Several assays are now available and commonly used to assess platelet reactivity. The oldest and most established assay is the Light transmission aggregometry (LTA) assay, often valued as the gold standard. This assay evaluates the response of the platelet to ADP through the function of both P2Y1 and P2Y12 receptors. Most studies use doses of 5-, 10-, or 20-µmol/L ADP as agonist, which is translated into an increase of light transmittance, reported as a percentage of Maximal Platelet Aggregation (MPA). High residual platelet reactivity (HRPR) with clopidogrel treatment using this technique has been associated with recurrent ischemic events (6,7). LTA has been criticized however for its poor reproducibility and lack of specificity for the P2Y12 pathway.
The other assays which are now very widely used, and are relatively easier to perform, are vasodilator stimulated phosphoprotein phosphorylation (VASP) analysis and the VerifyNow P2Y12 bedside assay.
The Vasodilator stimulated phosphoprotein (VASP) phosphorylation assay uses flow cytometry to measure the specific inhibition of the biochemical target of clopidogrel via the P2Y12 receptor. In patients treated with P2Y12 inhibitors, the phosphorylated state of VASP is a specific intracellular marker of residual P2Y12 receptor reactivity. This assay has the advantage of specifically assessing the P2Y12 receptor activity. The results are reported as a percentage value of Platelet Reactivity Index (PRI) (8). The platelet reactivity index VASP has also been associated with recurrent ischemic events after PCI (6,7) while having a strong negative predictive power below a certain cutoff (9). Unlike the measured aggregation induced by ADP, in VASP, the measurement in this assay does not include the contribution of the P2Y1 receptor to the platelet response.
The VerifyNow platelet function assay (Accumetrics, San Diego, California), a turbidimetric assay, is a very practical bedside tool that measures platelet aggregation to fibrinogen-coated beads in whole blood in response to an ADP induced stimulus. Reported as values of P2Y12 reaction units (PRU), a higher PRU value reflects a higher P2Y12 mediated platelet reactivity. A high PRU is associated with adverse cardiovascular events (7,10).
Many other platelet function assays are available, but since the abovementioned assays are the most commonly used ones in research trials and clinical practice, we will limit ourselves to those. Other available assays are: Multiplate Analyzer, PFA-100, whole blood thromboelastography (TEG) (6).
However, there is no consensus yet regarding the “gold standard” test, capable of defining resistance/poor response to clopidogrel. Indeed, while PRI VASP is the most specific test to assess the clopidogrel induced activation of the platelets, most clinical studies linking low response to clopidogrel with clinical outcomes have been performed with LTA or Verify Now (7), though more and more clinical data using the PRI VASP are being published.
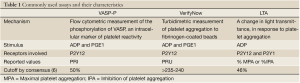
Given the lack of universal cutoff values, Bonello et al., 2010, defined consensus values for HTPR, based on prior studies, for the most commonly used platelet function assays (6) (Table 1). HTPR values were defined by ROC analyses as follows: (I) PRI >50% by VASP-P analysis; (II) >235 to 240 P2Y12 reaction units by VerifyNow P2Y12 assay; (III) >46% maximal for a 5-µmol/L ADP-induced aggregation; and (IV) >468 arbitrary aggregation units/min in response to ADP by Multiplate analyzer (6). While the authors argued against a routine use of those assays in any PCI patient, they reckoned its value in guiding personalized therapy for patients undergoing high-risk PCI or those with a known history of stent thrombosis.
Full Table
They also argued that there may not be one universal cutoff value defining HTPR for each assay, considering that those values may have different weights in different settings, like urgent versus elective PCI, periprocedural setting versus maintenance treatment phase. Moreover, even though these cutoff values had very good negative predictive values for recurrence of ischemic events, their positive predictive value was low for all used assays. The study deemed that, although HTPR is a major risk factor for future thrombotic events, it is nonetheless not the only culprit responsible for these.
In a separate study, Breet et al. showed that most used platelet reactivity assays were only modest predictors of outcomes at 1 year follow up, advocating against routine use of platelet reactivity assays in the low-risk group of patients undergoing PCI (11,12).
Agreement among tests
Gaglia et al. attempted to evaluate the degree of agreement and correlation among VASP-P, LTA and VerifyNow in patients on clopidogrel therapy undergoing PCI (8). The objective was to assess platelet reactivity between 6 to 24 hours following PCI and a minimum of six hours after a loading dose of clopidogrel.
Threshold values for HPR were used according to the latest consensus recommendations (6). There were considerable differences in the proportion of patients defined as having HTPR, as follows: 39.3% with VASP, 27.3% with VerifyNow, 23.1% with LTA ADP 5 µM, and 16.2% with LTA ADP 20 µM. The weakest correlation was noted between VASP and LTA ADP 5 µM (κ=0.33, 95% CI: 0.19-0.47). It was concluded that there was at best only a fair degree of agreement between those tests and that ‘platelet reactivity’ is not an interchangeable term to be used among the different assays used, even when abiding with “consensus” guidelines for cutoff values for each assay. Previous studies also had yielded similar observations, however with more disparate conclusions.
Nevertheless, even though agreement among these tests may be modest, it still remains that each one of the above-mentioned tests has shown, in separate studies, a significant correlation with occurrence of adverse cardiovascular outcomes beyond a certain cutoff value adopted by consensus. Therefore any of those tests can be used reliably to prognosticate about risk stratification of patients undergoing PCI.
Clopidogrel metabolism and influencing factors
Clopidogrel is an inactive pro-drug that requires oxidation by the hepatic cytochrome P450 (CYP) system in order to become the active metabolite. It has been observed that platelet inhibition response to clopidogrel is highly heritable (73%). Two sequential cytochrome P450-dependent oxidative steps are required to convert clopidogrel to its active metabolite. The first step leads to the formation of 2-oxo-clopidogrel. This metabolite is then metabolized to the active metabolite (6). This accounts for 15% of the drug metabolism. The remaining 85% is metabolized in the blood by esterases into an inactive metabolite and therefore does not contribute to drug effect. Cytochrome P450s enzymes are a large highly polymorphic family of mono-oxygenases. Some of the alleles coding for those enzymes have been reported to modify the concentration of some metabolites as well as the activity of some proteins, thereby modifying potential drug effect.
Of relevance, loss-of-function variants in the hepatic cytochrome 2C19 (CYP2C19) system have been reported to significantly alter the metabolism of clopidogrel and therefore its drug effect. While some alleles (like *17) would lead to an increased response to clopidogrel with increased bleeding risk, others, and most notoriously the *2 allele is related with a poorer response to clopidogrel. Others alleles, as well as combinations of mutated alleles with *2 deletion have been incriminated as well. However, genetically speaking, the highest risk profile group corresponds to those who are homozygous for allele *2 (13). Other studies have nevertheless identified heterozygous status as a significant risk (13-16). Being carrier of a loss-of-function allele is translated by a higher residual platelet reactivity, which in turn is associated with adverse outcomes, namely death, myocardial infarction and stent thrombosis as seen in the AFIJI study (15) and confirmed in a recent metanalysis (17). Nevertheless, despite the significant role of hereditary component for the clopidogrel responsiveness, only 18% of it explained by identified genetic mutations (6), suggesting that response to clopidogrel is the result of a very intricate genetic interplay.
Beside the genetic component, a controversial aspect is the drug-drug interaction at the level of the CYP3A4 enzyme activity, which is involved in the metabolism of clopidogrel into its active metabolite. This has been postulated mainly for statins metabolized by this enzyme, such atorvastatin and simvastatin (18,19). Nevertheless this has not confirmed by other studies (20,21). Similar risks have been identified about the concomitant use of proton pump inhibitors and clopidogrel (22). More so, there is also a variable intestinal absorption process, which could be affected by an ABCB1 gene polymorphism (7). As will be stated later in this review, some co morbid conditions are also associated with a poor platelet inhibition in response to clopidogrel, like Diabetes Mellitus, high BMI, and low ejection fraction (23-25). Among those, high BMI has been identified as an independent predictor of failure to overcome HTPR, even with clopidogrel dose adjustment (26).
Furthermore, platelet reactivity is not similar across all presentations of CAD, as we will see the studies reported in the review. A potential significant variable would be patient compliance to daily medication intake.
Does HRPR have any clinical significance?
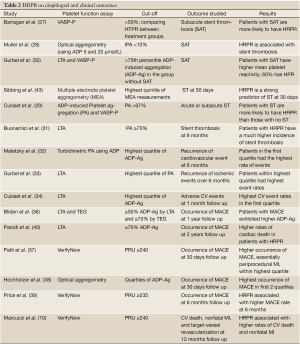
Stent thrombosis (Table 2)
It was noted that that patients experiencing subacute stent thrombosis have significantly higher platelet reactivity (27). Muller et al. reported in a cohort of 105 patients undergoing PCI that patients having stent thrombosis are more likely to be non-responders to clopidogrel (28), which was also noted by Cuisset et al. using two different assays (31). Analysis of the CREST study established HTPR and incomplete P2Y12 receptor inhibition as risk factors for subacute stent thrombosis (29). In 2007, it was observed that in patients undergoing PCI with DES placement, HRPR following a 600 mg loading dose of clopidogrel was associated with an increased risk of stent thrombosis at 6 months following PCI (32). Therefore, having a HRPR following a loading dose of clopidogrel is associated with a higher incidence of stent thrombosis.
Full Table
Recurrence of events (Table 2)
Matetzky et al. established that in patients with STEMI undergoing PCI, the higher the platelet aggregation (PA)as assessed by LTA (in the catheterization laboratory and then for 5 days following PCI), the higher the risk of sustaining a recurrent ischemic episode within 6 months (33), which was also confirmed by Gurbel et al. (34). This finding was extended to the NSTE-ACS population thanks to Cuisset et al., who demonstrated that in a cohort of 106 patients at 1 month follow up, patients within the highest PA quartile exhibited the highest rate of ischemic events (35). This trend is seen as early as the first few hours after PCI, with a higher incidence of periprocedural myocardial infarction (MI) among NSTE-ACS patients who demonstrate HRPR (41). The same conclusion has been drawn with patients undergoing angioplasty on elective basis, with those exhibiting HRPR on chronic clopidogrel therapy having a higher recurrence of events at 1 year (36).
A more recent study, the ARMYDA-PRO study (Antiplatelet therapy for Reduction of Myocardial Damage during Angioplasty-Platelet Reactivity Predicts Outcome), evaluated the use of point-of-care measurements of platelet inhibition in predicting clinical outcome in patients undergoing PCI, in a short-term follow-up (38). All patients had either received a 600 mg loading dose of clopidogrel or were on chronic clopidogrel therapy and all were maintained on aspirin and clopidogrel throughout the study. At 30-day follow up, those belonging to the highest quartile of platelet reactivity within the first 24 hours following PCI exhibited the highest rates of major adverse cardiac events (MACE) as defined by cardiac death, myocardial infarction (MI), target vessel revascularization. This difference was mainly driven by the risk of periprocedural MIs. The authors supported the use of a rapid point-of-care assay to monitor residual platelet reactivity after clopidogrel administration, which would identify the patients at a higher risk for MACE and potentiate the use of alternative antiplatelet regimen .A higher prevalence of MACE among patients with HRPR is also seen at 30-day follow up even after elective PCI (39).
These findings also hold true for the long term, with Price et al. noting that in patients with ACS undergoing PCI and DES placement, a HRPR following 600 mg loading-dose of clopidogrel was associated with a greater risk of cardiovascular death and stent thrombosis during a 6 months follow up (40). The higher risk incurred by HRPR also reflects on outcomes at 12 months follow up, with HRPR being predictive of MACE, as shown by Marcucci et al. (10).
In the RECLOSE 2-ACS study, Parodi et al. offered to study the role of HRPR as an independent prognostic indicator for occurrence of long term thrombotic events) (37). Prior big trials [like the GRAVITAS (Gauging Responsiveness with A VerifyNow assay — Impact on Thrombosis and Safety) study, to be discussed later] had not included the “sicker” population like those with NSTEMI and STEMI. Most studies only had short term follow up for clinical outcomes on patients with HRPR following PCI. The study included 1789 ACS patients, including all types of presentations ranging from unstable angina to STEMI. All patients were given 600 mg clopidogrel loading dose and 325 mg of aspirin upon presentation and maintained on daily doses of aspirin (325 mg/d) and clopidogrel (75 mg/d). However patients with HRPR were placed on 150-300 mg daily doses of clopidogrel or ticlodipine (500-1,000 mg/d) and supervised by ADP guidance, with a goal of reaching a reactivity value of less than 70% platelet aggregation measured by LTA. Platelet reactivity was assessed 12-18 hours after loading with clopidogrel, or 6 days later if patients were loaded with both clopidogrel and a IIB/IIIa inhibitor. The primary endpoint was a composite of cardiac death, myocardial infarction and urgent coronary revascularization, as well as stroke at two-year follow up. Follow up examinations throughout the study period showed that the primary endpoint occurred in 14.6% of HRPR group patients versus 8.7% of LRPR group, with an absolute risk reduction of 5.9% (95%CI: 1.6%-11.1%, P=0.003). The leading difference was concerning the rate of cardiac death (9.7% vs. 4.3%), while other components of the primary endpoint were similar between the 2 groups.
HRPR was associated with older age, previous history of MI, diabetes mellitus, hypercholesterolemia and low ejection fraction as well as congestive heart failure. That had also been established by the RECLOSE 1 study by Buonamici et al. in 2007 (32). Of note, both studies had higher proportion of STEMI in the low residual platelet reactivity group.
The RECLOSE 2-ACS study showed that while only 14% of patients undergoing PCI for ACS will have HRPR following a 600 mg clopidogrel loading dose, 38% of HRPR patients will still have HRPR even after adjusting antiplatelet therapy to HRPR status. Interestingly, it confirmed a previous finding seen with GRAVITAS that normalization/decrease of platelet reactivity after treatment is not translated into a better clinical outcome (42). The study confirmed that HRPR following 600 mg loading dose of clopidogrel as an independent prognostic marker of short and long-term ischemic events and is associated with increased risk ischemic events, both in the short and the long term, including stent thrombosis.
Caution is nonetheless in order as lower reactivity following a loading dose of clopidogrel has been associated with up to 4.5 fold increased risk in the 30-day incidence of major bleed (43). Patti et al. observed that this primary end point happened more frequently in patients within the lowest quartile of preprocedural PRU levels as compared to those in the highest quartile (10.1% vs. 1.3%, P=0.043), mainly due to entry-site hemorrhages. The optimal cutoff for the primary end point was a pre-PCI PRU value <189. Therefore, an enhanced response to clopidogrel may lead to with higher incidence of early major bleeding or entry-site complications in patients who undergo PCI.
High residual platelet reactivity and atherosclerotic burden and calcification
In a recent study, the correlation between high platelet reactivity (HPR) and its potential burden of atherosclerotic disease was investigated (44). Patients undergoing PCI according to guidelines (excluding STEMI) underwent pre-intervention volumetric intravascular ultrasound (IVUS) imaging, the gold standard modality to assess plaque atherosclerosis burden and calcification. Patients with HPR (>230 PRU) 16 hours following PCI and a first loading dose of clopidogrel had significantly greater calcification lengths, calcification arcs, and calcium indexes. Moreover, they had longer lesions and volumetric dimensions. Nonetheless plaque burden did not differ in the two groups. However after adjusting for univariate parameters, HRPR was found to be an independent predictor of the plaque findings. The study concluded that HPR is associated with a higher atherosclerotic burden and plaque calcification.
Different strategies to overcome high platelet reactivity
Is there a clinical benefit in double-dosing?
The CURRENT-OASIS 7 had ascertained that in patients with ACS following PCI, double-dose clopidogrel for at least the first 7 days was superior to standard-dose clopidogrel in reducing the incidence of a composite of cardiovascular death, myocardial infarction, or stroke and stent thrombosis at 1 month follow up (45). Moreover, a higher loading-dose of clopidogrel (600 vs. 300 mg) had been shown to achieve greater reduction in platelet reactivity in NSTE-ACS patient undergoing PCI (46). Nevertheless it was still unknown whether double-dosing clopidogrel maintenance therapy would have a clinical benefit in patients found to have HTPR.
The GRAVITAS randomized trial attempted to study the effect of high-dose clopidogrel versus standard-dose clopidogrel on platelet reactivity (42). This multicenter double blind trial enrolled 2,214 patients with stable CAD or non-ST-elevation ACS and HTPR 12 to 24 hours after undergoing PCI with drug-eluting stents. The VerifyNow assay, with a cutoff PRU≥230 was used to identify high platelet reactivity. Patients with HTPR were given high-dose platelet (600 mg loading dose and 150 mg daily doses) versus a placebo loading dose and then 75 mg daily. 586 patients were also selected form a cohort of 3,215 patients with no HTPR and were followed throughout the study. These were administered a standard daily dose of clopidogrel. Meanwhile all patients concomitantly received Aspirin doses ranging from 75 to 162 mg daily. Follow up visits and VerifyNow assay were subsequently performed at 30 days and 6 months.
The primary endpoint was the 6-month incidence of a composite of death from cardiovascular causes, nonfatal myocardial infarction, or stent thrombosis. There was a significantly higher reduction in on-treatment reactivity at 30 days and at 6 months with high dose clopidogrel than with standard-dose clopidogrel (80 PRU vs. 37 PRU with P<0.0001 and 85 PRU vs. 44 PRU; P<0.001, respectively). There was no difference in the rate of discontinuation of study drug due to GUSTO (Global Utilization of Streptokinase and t-PA for Occluded Coronary Arteries) severe or moderate bleeding across all 3 groups. High-dose clopidogrel led to an absolute 22% (95% CI: 18-26% and 24%, 95%CI: 20-28%) lower rate of HTPR compared with standard-dose clopidogrel at 30 days and 6 months. However this was not translated into a benefit in the primary endpoint at 6 month follow up with similar occurrence rates [2.3% vs. 2.3%; hazard ratio (HR), 1.01; 95% CI: 0.58-1.76; P=0.97].The primary endpoint occurred more frequently in patients with HTPR as opposed to those without (586 patients), although this did not reach statistical significance [25 (2.3%) vs. 8 (1.4%); HR, 1.68; 95% CI: 0.76-3.72; P=0.20].In other words, the study reconfirmed the association between HTPR and adverse cardiovascular events. Important limitations of the study were the fact that the study did not observe a high enough number of events to reach its targeted power of detecting 50% relative risk reduction following the study intervention. To note, the study population excluded the highest risk patients, namely those with NSTEMI and STEMI. This could potentially limit the use of this data for the abovementioned higher risk populations. Lastly, the baseline characteristics (and comorbidities) of patients with HTPR and no HTPR differed greatly, and the analysis was not adjusted for these differences.
The GRAVITAS study showed that despite significantly reducing platelet reactivity, therapy with high-dose clopidogrel following a loading dose in patients with HTPR is not translated into a reduction of primary endpoint at 6 months follow up.
Tailored clopidogrel loading?
Bonello et al. hypothesized that controlling residual platelet reactivity via tailored clopidogrel loading-doses would decrease the incidence of stent thrombosis (47). In a multicenter study, 429 patients with poor clopidogrel response after a 600-mg loading dose undergoing PCI (VASP index ≥50%) were randomized to a control group [214] and to a vasodilator- stimulated phosphoprotein (VASP)-guided group [215]. In the VASP-guided group, patients received up to 3 additional 600-mg loading doses of clopidogrel in order to achieve a VASP index <50% before PCI. The primary end point was the rate of stent thrombosis at 1 month. The secondary end points were the rates of major adverse cardiovascular events (MACE) and bleeding. There was a significantly lower rate of stent thrombosis at 1 month in the VASP-guided group (0.5% vs. 4.2%, P<0.01). The rate of MACE was also higher in the control group (8.9% vs. 0.5%, P<0.001). The rate of bleeding was similar in both groups. Of relevance, even after a 2,400-mg loading dose of clopidogrel, 8% of patients in the VASP-guided remained resistant to clopidogrel. The authors concluded that tailoring the clopidogrel loading doses according to platelet reactivity monitoring decreases the rate of early stent thrombosis after PCI without increasing bleeding.
In a similar study (48), Bonello et al. demonstrated that 86% of patients with clopidogrel resistance achieve a target VASP index <50% after receiving additional boluses of clopidogrel and demonstrate a lower rate of MACE occurrence at 1 month follow up, with similar rates of bleeding.
In view of the current evidence, it is safe to say that this strategy reduces the rate of major cardiac events and stent thrombosis in the short term without increasing the risk of bleeding, pending further studies to prove its sustained benefit in the long term.
Furthermore Bonello et al. (49) demonstrated that increasing loading doses of clopidogrel in NSTE-ACS patients with CY2C19*2 loss-of-function polymorphism, could achieve a reduction of platelet reactivity to 50% PRI. In contrast, Cuisset et al. showed that only a small portion of carriers of the CYP2C19*2 allele would show a significant reduction of HRPR even with 600 mg loading-doses and 150 mg daily doses (50).
Therefore it is safe to conclude that at present no consensus exists as to the impact of platelet function assays on predicting outcome with perhaps some impact on stent thrombosis.
A newer thienopyridine: prasugrel
With growing evidence concerning the limitations of clopidogrel therapy and clinically severe outcomes with recurrence of ischemic insults, researchers have focused their attention on developing new agents that would achieve a faster and higher degree of platelet inhibition.
One of those agents is prasugrel, a third generation thienopyridine agent. It is a prodrug that requires conversion to an active metabolite which will bind to the P2Y12 receptor and inhibit platelet activity. At currently used doses, prasugrel inhibits ADP – induced platelet aggregation more rapidly, more consistently, and has a higher potency when compared to both standard and higher doses of clopidogrel among healthy volunteers as well as patients with stable CAD (51,52). Moreover, a 60mg loading dose of prasugrel achieves higher and more consistent levels of the active metabolite than those achieved with a 300 mg loading dose of clopidogrel (51). Pharmacodynamics show that the degree of inhibition of platelet aggregation achieved with prasugrel within 30 minutes after treatment is comparable to the peak effect of clopidogrel 6 hours after administration (51), thus bypassing the need of prolonged pretreatment before PCI to achieve a clinical benefit.
The PRINCIPLE-TIMI 44 trial proved the greater degree of inhibition of platelet aggregation achieved by prasugrel (60 mg loading dose followed by 10 mg daily) as compared to high-dose clopidogrel (600 mg loading dose then 150 mg daily) (53). The study populations were patients presenting with angina within the last 14 days and planned for PCI. This effect was seen as early as 30 minutes after administration of the drug, and was sustained during the maintenance dose, as ascertained by LTA platelet assay, and confirmed by VerifyNow and VASP-P assays. The authors concluded that prasugrel, as compared to high-dose clopidogrel, achieves an earlier, greater and sustained platelet inhibition. Nevertheless the study was not powered to study clinical outcomes.
Overcoming the barriers of genotype
With the known limitations of clopidogrel among CYP2C19*2 carriers (50), the RAPID GENE study (54) explored the effect of prasugrel therapy (versus standard clopidogrel therapy) on HRPR following PCI for ACS (excluding STEMI patients) or stable angina. The patients were randomly assigned to rapid point-of-care genotyping or to standard treatment. People in the rapid genotyping group underwent screening for the CYP2C19*2 allele via a novel point-of-care genetic test for the CYP2C19*2 allele with a buccal swab. Those found to be carriers were started on 10mg prasugrel daily, while non-carriers as well as patients in the standard treatment group were placed on 75 mg clopidogrel daily. All patients received 600 mg of clopidogrel at least 24 hours before PCI. All patients were tested for a baseline platelet reactivity immediately after PCI and had not significantly different PRU values at baseline, with CYP2C19*2 carriers having higher PRU compared to non-carriers.
The primary endpoint was the proportion of CYP2C19*2 carriers with HTPR (with a (PRU) value >234 using the VerifyNow assay), after 1 week of dual antiplatelet treatment, assuming that stabilization of platelet inhibition by clopidogrel and prasugrel is achieved by that time. There was a significant decrease in the rate of HTPR in CYP2C19*2 carriers in the rapid genotype testing group compared to those receiving standard treatment. The authors concluded that rapid genetic testing followed by a personalized treatment reduces the number of CYP2C19*2 carriers patients undergoing PCI who would still have HTPR. They added that the use of this kind of test can identify a population at increased risk of adverse ischemic events and overcome it via the use of prasugrel. However, a major limitation of the study was that a decrease of platelet reactivity was used as the surrogate for clinical benefit, assuming that HTPR is equivalent to increased risk of MACE.
Clinical superiority of prasugrel
The first trial to assess the clinical outcomes of prasugrel was the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction (TRITON-TIMI) 38 (55). 13,608 patients presenting with moderate- to-high- risk ACS syndromes for scheduled PCI were randomly assigned to receive prasugrel (a loading dose of 60 mg and then 10 mg/day as maintenance dose) or clopidogrel (300 mg loading dose, followed by 75 mg/day) for a period of 6 to 15 months. All patients concomitantly received aspirin throughout the study.
The results of the study showed a significant difference in the occurrence of the primary end point (death from cardiovascular causes, nonfatal myocardial infarction or nonfatal stroke over the next 15 months) with 9.9% in the prasugrel group versus 12.1% in the clopidogrel group, and a hazard ratio for prasugrel vs. clopidogrel of 0.81 (95% CI: 0.73 to 0.90; P<0.001). Similarly, there was a significant reduction in the rate of myocardial infarction with 9.7% for clopidogrel vs. 7.4% for prasugrel (P<0.001), urgent target-vessel revascularization (3.7% vs. 2.5%; P<0.001), as well as stent thrombosis (2.4% vs. 1.1%; P<0.001). Nevertheless, there was a higher propensity for major bleed in the prasugrel group, with an incidence of 2.4% of patients on prasugrel vs. 1.8% of patients receiving clopidogrel (hazard ratio, 1.32; 95% CI: 1.03 to 1.68; P=0.03). Moreover, the rate of life-threatening bleeding was also higher (1.4% vs. 0.9%; P=0.01), as well as nonfatal bleeding (1.1% vs. 0.9%; hazard ratio, 1.25; P=0.23) and fatal bleeding (0.4% vs. 0.1%; P=0.002).
While the rapid onset of action with prasugrel may explain the reduction in the rate of early MI (before day 3) observed in this trial, this significant reduction in the rate of endpoints persisted even after 3 days (assuming both drugs would have achieved peak effect), proving the continued benefit and superiority of prasugrel over clopidogrel during maintenance therapy. The estimated number needed to treat to prevent one primary endpoint with prasugrel at the studied dosage, compared with standard-dose clopidogrel, during a 15-month period was 46 patients. On the other hand, the number needed to harm, as defined by non-CABG-related TIMI major hemorrhage was 167 patients. The authors concluded that prasugrel does reduce the rate of recurrence of ischemic events in patients with ACS undergoing scheduled PCI, however at the expense of an increase in the incidence of major bleeding. Hence, contraindications for the use of prasugrel are: age older than 75 years old, weight less than 60 kg (with a recommended decreased dose of 5 mg, though no efficacy is proven), history of TIA or stroke or pathologic active bleed (56).
However, even though prasugrel achieves greater platelet inhibition and is superior to clopidogrel in preventing ischemic events, resistant platelet reactivity while on prasugrel therapy still remains a cause of concern. It was found that in patients undergoing PCI in the setting of ACS who receive prasugrel, 25.2% would still maintain HTPR (as defined VASP index >50%) after 60 mg loading dose (57). It was also found that these patients have higher incidence of MACE during the first month.
In a small study including 80 patients, Lhermusier et al. studied the usefulness of prasugrel reloading in ACS patients planned for PCI who had already received a 300 mg loading dose of clopidogrel (58). Various doses of prasugrel loading were used (10, 30, 60 mg) at least 3 hours after the clopidogrel dose. The assays used were VASP-P with cutoff PRI >60% and VerifyNow with a cutoff PRU>235). Although nearly all patients achieved the desired inhibition, 30 mg was the most adequate reloading dose in achieving the desired platelet inhibition with an efficacy sustained for 12-18 hours. Although no major TIMI bleed was seen in the 3 groups, the study did not assess any clinical outcomes or safety parameters.
Switching from prasugrel to clopidogrel remains a valid option for ACS patients even during the maintenance clopidogrel therapy. The SWAP (Switching Antiplatelet)study demonstrated further degree of platelet inhibition achieved once patients are switched to prasugrel, with or without a loading dose of prasugrel (59). This benefit was observed as early as 2 hours after a loading dose of prasugrel.
Ticagrelor: a step further?
Ticagrelor, also known as AZD6140, is a novel oral P2Y12 receptor antagonist that reversibly binds to the P2Y12 receptor, thus preventing the binding of ADP. This new drug does not require metabolic activation, in order to exert its effect. Among patients with stable CAD, ticagrelor showed a greater degree of platelet inhibition compared to clopidogrel (60,61). The inhibitory effect of 180 mg loading dose of ticagrelor is superior to that achieved by 600 mg clopidogrel. More so, its inhibitory effect weans faster than with clopidogrel (61).
These findings were extended to the NSTE-ACS population with the DISPERSE (Dose confirmation Study assessing anti-Platelet Effects of AZD6140 vs. clopidogrel in non-ST-segment Elevation myocardial infarction)-2 study (62). The study compared different doses of AZD6140 (90 and 180 mg twice daily) with clopidogrel in patients with presenting with NSTE-ACS. It showed that ticagrelor exerts a greater platelet inhibitory effect compared to clopidogrel in ACS patients. This finding was noted both during maintenance therapy and in the early hours of treatment.
The Study of Platelet Inhibition and Patient Outcomes (PLATO), compared ticagrelor (with a 180 mg loading dose, then 90 mg twice daily) and clopidogrel (300-to-600-mg loading dose, then 75 mg daily) among 18,624 patients admitted to the hospital with an ACS, including STEMI, for the prevention of cardiovascular events in this population (63). Problems encountered with ticagrelor are dose-related (i.e. more with 180 than 90 mg) and consist of episodes of dyspnea and ventricular pauses on Holter monitoring. All patients also received daily aspirin for the study period. The occurrence of the primary end point (time to occurrence of a composite of death from vascular causes, myocardial infarction, or stroke) was significantly less in the ticagrelor group than in the clopidogrel group (9.8% of patients vs. 11.7% at 12 months follow up; HR of 0.84; 95% CI: 0.77 to 0.92; P<0.001). The difference in treatment effect was noticeable from the first 30 days of treatment and sustained till the end of the study period. Similar reductions were seen with the ticagrelor group in regards to the rates of the composite end point of death from any cause, MI, or stroke (10.2% vs. 12.3%, P<0.001) as well as in the rate of death from any cause with ticagrelor as well (4.5%, vs. 5.9%; P<0.001).
The rate of stroke was comparable in both treatment groups, although more hemorrhagic strokes occurred with ticagrelor than with clopidogrel [23 (0.2%) vs. 13 (0.1%), nominal P=0.10]. Among patients who underwent invasive treatment, the rate of the primary end point was also lower with ticagrelor (8.9% vs. 10.6%; P=0.003).There was a lower incidence of definite stent thrombosis in the ticagrelor group than in the clopidogrel group (1.3% vs. 1.9%, P=0.009).
The rates of major bleeding were not significantly different between ticagrelor and clopidogrel groups whether using the criteria defined in the trial (P=0.43) or the Thrombolysis in Myocardial Infarction (TIMI) criteria (P=0.57). Neither was there a difference in fatal or life-threatening bleeding (5.8% in both groups, P=0.70). However, in the ticagrelor group, there was a higher rate of non-CABG-related major bleeding according to the study criteria (4.5% vs. 3.8%, P=0.03) and the TIMI criteria (2.8% vs. 2.2%, P=0.03).Moreover, the ticagrelor group had a higher incidence of intracranial bleeding [26 (0.3%) vs. 14 (0.2%), P=0.06], including fatal intracranial bleeding [11 (0.1%) vs. 1 (0.01%), P=0.02]. However, there were fewer episodes of other types of fatal bleeding in the ticagrelor group [9 (0.1%), vs. 21 (0.3%) in the clopidogrel group; P=0.03]. Notable side effects of ticagrelor during the study were dyspnea and asymptomatic ventricular pauses in the first week. Of note, ticagrelor use also had a higher incidence of creatinine and uric acid increase.
A significant advantage which ticagrelor possesses as opposed to thienopyridines is that its action is reversible, which may be a very significant parameter for control of bleeding.
The PLATO study shows us that in patients ACS with or without ST-segment elevation, treatment with ticagrelor, compared to clopidogrel, significantly reduces the rate of death from vascular causes, MI, or stroke, without increasing the overall rate of major bleeding with a sustained benefit throughout the study period (360 days), nevertheless with an increase in the rate of non–procedure-related bleeding.
A PLATO substudy by Storey et al. (64) showed that ticagrelor achieves a greater inhibitory response than clopidogrel with the mean maximum LTA responses (using ADP 20 µM) post maintenance dose of 44±15% for clopidogrel and 28±10% for ticagrelor (P<0.001). High platelet reactivity was seen more frequently with clopidogrel than ticagrelor following a loading dose and during the maintenance therapy. The conclusion was that in patients with ACS ticagrelor achieves greater platelet inhibition, starting in the early hours following loading, with a sustained effect throughout maintenance phase.
Ticagrelor versus prasugrel
Most recently, Alexopoulos et al. proposed to compare the antiplatelet effects of ticagrelor and prasugrel in patients with high on-clopidogrel platelet reactivity 24 hours following PCI (65). 44 patients with HTPR, PRU ≥235, were randomized to either 10mg daily dose of prasugrel or 90 mg twice daily of ticagrelor. After 15 days of treatment, a crossover was performed from each treatment group to the other. The primary endpoint was platelet reactivity assessed at 2 different times (the first was pre-crossover, the second was post-crossover).
The results were heavily in favor of ticagrelor as far the primary endpoint was concerned. At the end of the 2 treatment periods PR was lower with ticagrelor (32.9 PRU, 95% CI: 18.7 to 47.2) compared with prasugrel (101.3 PRU, 95% CI: 86.8 to 115.7) with a mean difference in least squares of –68.3 PRU (95% CI: –88.6 to –48.1; P<0.001). The secondary endpoint, which was the rate of HTPR, was found to be 0% for ticagrelor versus 2.4% for prasugrel (1 of 42, P=0.5).
No patients had any MACE in either study group during the study period, and no TIMI major bleed was observed in either group. The study was not powered to assess the association between the primary endpoint and clinical outcomes. The study shows that ticagrelor achieves a significantly greater platelet inhibition as compared to prasugrel in ACS patients with HTPR 24 hours post PCI while on clopidogrel undergoing PCI. It also shows that patients on prasugrel can directly be switched to clopidogrel and vice versa (65).
Modifying HTPR with adjunctive antiplatelet therapy
The impact of adjuvant antiplatelet or anticoagulant on patients with HRPR following clopidogrel loading dose in the PCI setting was studied. The ISAR-REACT 4 (Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment-4) study looked at the efficacy of abciximab plus unfractionated heparin versus bivalirudin in NSTEMI patients undergoing urgent PCI who had HPR following a loading-dose of clopidogrel (66). The study showed no difference in the rate of occurrence of the primary combined efficacy endpoint (death, any recurrent MI, urgent target-vessel revascularization) during a 30-day follow-up period. Nevertheless, bivalirudin showed a significantly reduced risk of major bleeding.
In the ISAR- REACT4 substudy, Sibbing et al. showed that for patients having received abciximab with Heparin, there was no difference in the incidence of the efficacy endpoint in HPR versus no-HPR patients (9.4% vs. 6.7%; OR: 1.4; 95% CI: 0.6 to 3.5; P=0.43) (67). However, for bivalirudin, there was a significantly higher incidence of the efficacy endpoint in HPR versus no-HPR patients (22.0% vs. 5.0%; odds ratio: 5.4; 95% CI: 2.4 to 12.1; P<0.0001). They concluded that the impact that HPR has on clinical outcomes may be determined by the type of adjunctive antithrombotic therapy that was used during PCI.
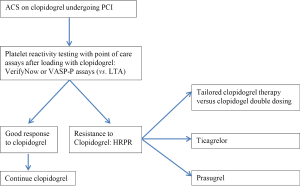
Conclusions (Figure 1)
While platelet reactivity is not routinely tested in ACS patients undergoing PCI, HRPR to clopidogrel is a clinical conundrum for interventional cardiologists. Despite the fact that higher doses of clopidogrel have shown some benefit in that regard, the choice of alternative agents may be the preferred practice. Recent guidelines have upgraded prasugrel and ticagrelor as superior alternatives to clopidogrel in STEMI (68) as well as in Unstable Angina/NSTEMI (69).Weighing the risk of bleeding is clearly required under these circumstances. At present, prasugrel use only applies to patients undergoing PCI, while the twice daily dosing with ticagrelor raises an issue of patient compliance. In addition, these drugs are a more expensive and less available option in many countries. Nevertheless, these two potent antiplatelets provide a breakthrough against the poor response to clopidogrel and may soon replace clopidogrel in routine clinical practice.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Fox KA, Mehta SR, Peters R, et al. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation 2004;110:1202-8.
- Sabatine MS, Cannon CP, Gibson CM, et al. Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA 2005;294:1224-32.
- Smith SC Jr, Feldman TE, Hirshfeld JW Jr, et al. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update 2001 Guidelines for Percutaneous Coronary Intervention). Circulation 2006;113:e166-286.
- Price MJ, Angiolillo DJ, Teirstein PS, et al. Platelet reactivity and cardiovascular outcomes after percutaneous coronary intervention: a time-dependent analysis of the Gauging Responsiveness with a VerifyNow P2Y12 assay: Impact on Thrombosis and Safety (GRAVITAS) trial. Circulation 2011;24:1132-7.
- Price MJ, Berger PB, Angiolillo DJ, et al. Evaluation of individualized clopidogrel therapy after drug-eluting stent implantation in patients with high residual platelet reactivity: design and rationale of the GRAVITAS trial. Am Heart J 2009;157:818-24, 824.e1.
- Bonello L, Tantry US, Marcucci R, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol 2010;56:919-33.
- Cuisset T, Morange PE, Alessi MC. Recent advances in the pharmacogenetics of clopidogrel. Hum Genet 2012;131:653-64.
- Gaglia MA, Torguson R, Pakala R, et al. Correlation between light transmission aggregometry, VerifyNow P2Y12, and VASP-P platelet reactivity assays following percutaneous coronary intervention. J Interv Cardiol 2011;24:529-34.
- Bonello L, Paganelli F, Arpin-Bornet M, et al. Vasodilator-stimulated phosphoprotein phosphorylation analysis prior to percutaneous coronary intervention for exclusion of postprocedural major adverse cardiovascular events. Journal of thrombosis and haemostasis. J Thromb Haemost 2007;5:1630-6.
- Marcucci R, Gori AM, Paniccia R, et al. Cardiovascular death and nonfatal myocardial infarction in acute coronary syndrome patients receiving coronary stenting are predicted by residual platelet reactivity to ADP detected by a point-of-care assay: a 12-month follow-up. Circulation 2009;119:237-42.
- Breet NJ, van Werkum JW, Bouman HJ, et al. Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. JAMA 2010;303:754-62.
- ten Berg JM. Error in a study of the comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary artery stent implantation. JAMA 2011;305:2172-3.
- Simon T, Verstuyft C, Mary-Krause M, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med 2009;360:363-75.
- Frere C, Cuisset T, Morange PE, et al. Effect of cytochrome p450 polymorphisms on platelet reactivity after treatment with clopidogrel in acute coronary syndrome. Am J Cardiol 2008;101:1088-93.
- Collet JP, Hulot JS, Pena A, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet 2009;373:309-17.
- Sibbing D, Stegherr J, Latz W, et al. Cytochrome P450 2C19 loss-of-function polymorphism and stent thrombosis following percutaneous coronary intervention. Eur Heart J 2009;30:916-22.
- Hulot JS, Collet JP, Silvain J, et al. Cardiovascular risk in clopidogrel-treated patients according to cytochrome P450 2C19*2 loss-of-function allele or proton pump inhibitor coadministration: a systematic meta-analysis. J Am Coll Cardiol 2010;56:134-43.
- Lau WC, Waskell LA, Watkins PB, et al. Atorvastatin reduces the ability of clopidogrel to inhibit platelet aggregation: a new drug-drug interaction. Circulation 2003;107:32-7.
- Neubauer H, Gunesdogan B, Hanefeld C, et al. Lipophilic statins interfere with the inhibitory effects of clopidogrel on platelet function--a flow cytometry study. Eur Heart J 2003;24:1744-9.
- Serebruany VL, Midei MG, Malinin AI, et al. Absence of interaction between atorvastatin or other statins and clopidogrel: results from the interaction study. Arch Intern Med 2004;164:2051-7.
- Mitsios JV, Papathanasiou AI, Rodis FI, et al. Atorvastatin does not affect the antiplatelet potency of clopidogrel when it is administered concomitantly for 5 weeks in patients with acute coronary syndromes. Circulation 2004;109:1335-8.
- Ho PM, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA 2009;301:937-44.
- Angiolillo DJ, Fernández-Ortiz A, Bernardo E, et al. Platelet function profiles in patients with type 2 diabetes and coronary artery disease on combined aspirin and clopidogrel treatment. Diabetes 2005;54:2430-5.
- Angiolillo DJ, Fernández-Ortiz A, Bernardo E, et al. Platelet aggregation according to body mass index in patients undergoing coronary stenting: should clopidogrel loading-dose be weight adjusted? J Invasive Cardiol 2004;16:169-74.
- Bernlochner I, Byrne RA, Kastrati A, et al. The future of platelet function testing to guide therapy in clopidogrel low and enhanced responders. Expert Rev Cardiovasc Ther 2011;9:999-1014.
- Bonello-Palot N, Armero S, Paganelli F, et al. Relation of body mass index to high on-treatment platelet reactivity and of failed clopidogrel dose adjustment according to platelet reactivity monitoring in patients undergoing percutaneous coronary intervention. Am J Cardiol 2009;104:1511-5.
- Barragan P, Bouvier JL, Roquebert PO, et al. Resistance to thienopyridines: clinical detection of coronary stent thrombosis by monitoring of vasodilator-stimulated phosphoprotein phosphorylation. Catheter Cardiovasc Interv 2003;59:295-302.
- Müller I, Besta F, Schulz C, et al. Prevalence of clopidogrel non-responders among patients with stable angina pectoris scheduled for elective coronary stent placement. Thromb Haemost 2003;89:783-7.
- Gurbel PA, Bliden KP, Samara W, et al. Clopidogrel effect on platelet reactivity in patients with stent thrombosis: results of the CREST Study. J Am Coll Cardiol 2005;46:1827-32.
- Sibbing D, Braun S, Morath T, et al. Platelet reactivity after clopidogrel treatment assessed with point-of-care analysis and early drug-eluting stent thrombosis. J Am Coll Cardiol 2009;53:849-56.
- Cuisset T, Frere C, Quilici J, et al. Predictive values of post-treatment adenosine diphosphate-induced aggregation and vasodilator-stimulated phosphoprotein index for stent thrombosis after acute coronary syndrome in clopidogrel-treated patients. Am J Cardiol 2009;104:1078-82.
- Buonamici P, Marcucci R, Migliorini A, et al. Impact of platelet reactivity after clopidogrel administration on drug-eluting stent thrombosis. J Am Coll Cardiol 2007;49:2312-7.
- Matetzky S, Shenkman B, Guetta V, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation 2004;109:3171-5.
- Gurbel PA, Bliden KP, Guyer K, et al. Platelet reactivity in patients and recurrent events post-stenting: results of the PREPARE POST-STENTING Study. J Am Coll Cardiol 2005;46:1820-6.
- Cuisset T, Frere C, Quilici J, et al. High post-treatment platelet reactivity identified low-responders to dual antiplatelet therapy at increased risk of recurrent cardiovascular events after stenting for acute coronary syndrome. J Thromb Haemost 2006;4:542-9.
- Bliden KP, DiChiara J, Tantry US, et al. Increased risk in patients with high platelet aggregation receiving chronic clopidogrel therapy undergoing percutaneous coronary intervention: is the current antiplatelet therapy adequate? J Am Coll Cardiol 2007;49:657-66.
- Parodi G, Marcucci R, Valenti R, et al. High residual platelet reactivity after clopidogrel loading and long-term cardiovascular events among patients with acute coronary syndromes undergoing PCI. JAMA 2011;306:1215-23.
- Patti G, Nusca A, Mangiacapra F, et al. Point-of-care measurement of clopidogrel responsiveness predicts clinical outcome in patients undergoing percutaneous coronary intervention results of the ARMYDA-PRO (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty-Platelet Reactivity Predicts Outcome) study. J Am Coll Cardiol 2008;52:1128-33.
- Hochholzer W, Trenk D, Bestehorn HP, et al. Impact of the degree of peri-interventional platelet inhibition after loading with clopidogrel on early clinical outcome of elective coronary stent placement. J Am Coll Cardiol 2006;48:1742-50.
- Price MJ, Endemann S, Gollapudi RR, et al. Prognostic significance of post-clopidogrel platelet reactivity assessed by a point-of-care assay on thrombotic events after drug-eluting stent implantation. Eur Heart J 2008;29:992-1000.
- Cuisset T, Frere C, Quilici J, et al. High post-treatment platelet reactivity is associated with a high incidence of myonecrosis after stenting for non-ST elevation acute coronary syndromes. Thromb Haemost 2007;97:282-7.
- Price MJ, Berger PB, Teirstein PS, et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA 2011;305:1097-105.
- Patti G, Pasceri V, Vizzi V, et al. Usefulness of platelet response to clopidogrel by point-of-care testing to predict bleeding outcomes in patients undergoing percutaneous coronary intervention (from the Antiplatelet Therapy for Reduction of Myocardial Damage During Angioplasty-Bleeding Study). Am J Cardiol 2011;107:995-1000.
- Chirumamilla AP, Maehara A, Mintz GS, et al. High platelet reactivity on clopidogrel therapy correlates with increased coronary atherosclerosis and calcification: a volumetric intravascular ultrasound study. JACC Cardiovasc Imaging 2012;5:540-9.
- Mehta SR, Tanguay JF, Eikelboom JW, et al. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet 2010;376:1233-43.
- Cuisset T, Frere C, Quilici J, et al. Benefit of a 600-mg loading dose of clopidogrel on platelet reactivity and clinical outcomes in patients with non-ST-segment elevation acute coronary syndrome undergoing coronary stenting. J Am Coll Cardiol 2006;48:1339-45.
- Bonello L, Camoin-Jau L, Armero S, et al. Tailored clopidogrel loading dose according to platelet reactivity monitoring to prevent acute and subacute stent thrombosis. Am J Cardiol 2009;103:5-10.
- Bonello L, Camoin-Jau L, Arques S, et al. Adjusted clopidogrel loading doses according to vasodilator-stimulated phosphoprotein phosphorylation index decrease rate of major adverse cardiovascular events in patients with clopidogrel resistance: a multicenter randomized prospective study. J Am Coll Cardiol 2008;51:1404-11.
- Bonello L, Armero S, Ait Mokhtar O, et al. Clopidogrel loading dose adjustment according to platelet reactivity monitoring in patients carrying the 2C19*2 loss of function polymorphism. J Am Coll Cardiol 2010;56:1630-6.
- Cuisset T, Quilici J, Cohen W, et al. Usefulness of high clopidogrel maintenance dose according to CYP2C19 genotypes in clopidogrel low responders undergoing coronary stenting for non ST elevation acute coronary syndrome. Am J Cardiol 2011;108:760-5.
- Brandt JT, Payne CD, Wiviott SD, et al. A comparison of prasugrel and clopidogrel loading doses on platelet function: magnitude of platelet inhibition is related to active metabolite formation. Am Heart J 2007;153:66.e9-16.
- Jernberg T, Payne CD, Winters KJ, et al. Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin-treated patients with stable coronary artery disease. Eur Heart J 2006;27:1166-73.
- Wiviott SD, Trenk D, Frelinger AL, et al. Prasugrel compared with high loading- and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation 2007;116:2923-32.
- Roberts JD, Wells GA, Le May MR, et al. Point-of-care genetic testing for personalisation of antiplatelet treatment (RAPID GENE): a prospective, randomised, proof-of-concept trial. Lancet 2012;379:1705-11.
- Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001-15.
- Kushner FG, Hand M, Smith SC Jr, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2009;54:2205-41.
- Bonello L, Pansieri M, Mancini J, et al. High on-treatment platelet reactivity after prasugrel loading dose and cardiovascular events after percutaneous coronary intervention in acute coronary syndromes. J Am Coll Cardiol 2011;58:467-73.
- Lhermusier T, Voisin S, Mejean S, et al. Switching patients from clopidogrel to prasugrel in acute coronary syndrome: effects of prasugrel loading dose on residual platelet reactivity. J Thromb Haemost 2012;10:1946-9.
- Angiolillo DJ, Saucedo JF, Deraad R, et al. Increased platelet inhibition after switching from maintenance clopidogrel to prasugrel in patients with acute coronary syndromes: results of the SWAP (SWitching Anti Platelet) study. J Am Coll Cardiol 2010;56:1017-23.
- Husted S, Emanuelsson H, Heptinstall S, et al. Pharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: a double-blind comparison to clopidogrel with aspirin. Eur Heart J 2006;27:1038-47.
- Gurbel PA, Bliden KP, Butler K, et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation 2009;120:2577-85.
- Cannon CP, Husted S, Harrington RA, et al. Safety, tolerability, and initial efficacy of AZD6140, the first reversible oral adenosine diphosphate receptor antagonist, compared with clopidogrel, in patients with non-ST-segment elevation acute coronary syndrome: primary results of the DISPERSE-2 trial. J Am Coll Cardiol 2007;50:1844-51.
- Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045-57.
- Storey RF, Angiolillo DJ, Patil SB, et al. Inhibitory effects of ticagrelor compared with clopidogrel on platelet function in patients with acute coronary syndromes: the PLATO (PLATelet inhibition and patient Outcomes) PLATELET substudy. J Am Coll Cardiol 2010;56:1456-62.
- Alexopoulos D, Galati A, Xanthopoulou I, et al. Ticagrelor versus prasugrel in acute coronary syndrome patients with high on-clopidogrel platelet reactivity following percutaneous coronary intervention: a pharmacodynamic study. J Am Coll Cardiol 2012;60:193-9.
- Kastrati A, Neumann FJ, Schulz S, et al. Abciximab and heparin versus bivalirudin for non-ST-elevation myocardial infarction. N Engl J Med 2011;365:1980-9.
- Sibbing D, Bernlochner I, Schulz S, et al. Prognostic value of a high on-clopidogrel treatment platelet reactivity in bivalirudin versus abciximab treated non-ST-segment elevation myocardial infarction patients. ISAR-REACT 4 (Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment-4) platelet substudy. J Am Coll Cardiol 2012;60:369-77.
- Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC), Steg PG, James SK, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). Eur Heart J 2012;33:2569-619.
- Jneid H, Anderson JL, Wright RS, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/Non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2012;126:875-910.


