Association between serum adropin level and coronary artery disease: a systematic review and meta-analysis
Introduction
Coronary artery disease (CAD) is caused by atherosclerosis of the coronary arteries that leads to a restriction of blood flow to the heart (1). With the improvement of people’s living standards and lifestyle changes, the morbidity and mortality of CAD increase year by year, which seriously threatens the health of human beings (2).
Adropin, a secreted protein consisting of 76 amino acids with a molecular weight of 4,999.9 D, is encoded by energy balance related genes and is a new regulatory polypeptide involved in energy homeostasis (3,4). Some studies have found that adropin is associated with CAD, which can regulate lipid metabolism, improve insulin resistance, and protect vascular endothelial cell function and anti-inflammatory effect (5-7). However, the samples of these studies were quite small. Therefore, this study was performed to include related studies and systematically evaluated the relationship between serum adropin levels and CAD.
Methods
Guidelines and searches
This meta-analysis followed the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) Statement issued in 2009. Electronic databases, including the Cochrane Library, PubMed, Embase, Ovid, CBM, CNKI, VIP, WanFang Data databases were searched for relevant studies written in English or Chinese. The search terms include “adropin” “coronary disease” “coronary artery disease” “coronary heart disease”, “coronary and heart disease”. Take PubMed as an example, Table 1 shows its specific search strategy.

Full table
Study selection
All eligible studies satisfying the following criteria were initially included in the analysis: (I) type of study randomized controlled trials, cohort studies, case-control studies, regardless of whether distributional or blinding; (II) research object patients diagnosed with CAD, acute myocardial infarction (AMI), unstable angina pectoris (UAP), stable angina pectoris (SAP), and CAD-free healthy people; (III) observe the indicator serum adropin levels. The exclusion criteria were: (I) severe cardiovascular and cerebrovascular diseases, severe respiratory diseases, liver or kidney organ dysfunction; (II) no data or data is obviously wrong; and (III) for repeated publications, only the most complete data are included.
Data extraction and quality assessment
All the enrolled studies were independently reviewed by two investigators and any disagreements in quality assessments were resolved by discussion. Data extraction mainly include: (I) normal information, including title, first author’s name, date of publication and source of documentation; (II) study object characteristics, including age, sex, blood lipids, liver and kidney function, adropin levels and so on; (III) observative indicators, including serum adropin level in the CAD group and control group. The methodology quality evaluation of the study was carried out in reference to Cochrane Handbook 5.1.0.
Statistical analysis
Meta-analysis was performed using RevMan 5.2 software provided by the Cochrane Collaboration. Standard mean difference (SMD) with its 95% confidence interval (CI) was used as the effect size in this study. Heterogeneity included in the study results was analyzed using the χ2 test. To reduce the potential effect of statistical heterogeneity, random-effect model was chosen in this meta-analysis. Besides, subgroup analyzes are conducted to analyze the potential heterogeneity and characteristics in different patients.
Results
Literature search results
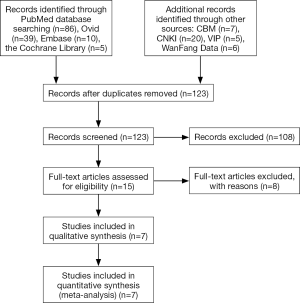
At the beginning, 178 papers were found. These studies were screened layer by layer. Finally, there were seven studies included (8-14). The literature screening process and results are shown in Figure 1.
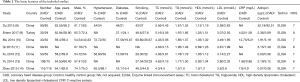
The basic characteristics of inclusion studies and methodological quality evaluation
The basic features of the study included in Table 2, the methodological quality evaluation results are attached.
Full table
Meta-analysis results
Serum adropin levels in CAD group and control group
A total of seven studies were included (8-14). Meta-analysis of random effects model showed that serum adropin level in CAD group was lower than that in control group (SMD =−2.44, 95% CI: −3.87 to −1.01), P=0.0008) (Figure 2).

The levels of serum adropin in AMI group and control group
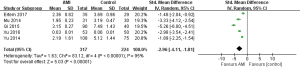
A total of five studies were included (9-13). Meta-analysis of random effects model showed that serum Adropin level in AMI group was lower than that in control group (SMD =−2.96, 95% CI: −4.11 to −1.81, P<0.00001) (Figure 3).
The levels of serum adropin in UAP group and control group
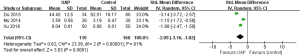
A total of three studies were included (8,10,12). Meta-analysis of random effects model showed that serum adropin level in UAP group was lower than that in control group (SMD =−2.09, 95% CI: −3.16 to −1.02, P=0.0001) (Figure 4).
The levels of serum adropin in SAP group and control group were compared
A total of two studies were included (8,13). Meta-analysis of random effects model showed that serum adropin level in SAP group was lower than that in control group (SMD =−1.23, 95% CI: −2.14 to −0.33, P=0.007) (Figure 5).

Quality evaluation
The study was biased using funnel charts and fail-safe number for evaluation. Funnel diagram was symmetrical, funnel-like, published bias is small. The fail-safe number of CAD is 2,981, high reliability. Based on the characteristics of the data collected, this study uses a one-by-one deletion of the included literature to conduct a sensitivity analysis to evaluate the impact of a single document on the overall outcome. This article analyzes the sensitivity of CAD and all subgroup. The results showed that there was no single document that could significantly influence the statistical results of original analysis and subgroup analysis. Indicating that the results excluded are less sensitive to the conclusion and the conclusion is more credible.
Discussion
We are the first meta-analysis of the relationship between adropin and CAD so far in the world. A total of 7 case-control studies were included in this meta-analysis (8-14), including 525 CAD patients and 420 healthy controls. The results of meta-analysis showed that the levels of serum adropin in patients with CAD, AMI, UAP and SAP were significantly lower than those in healthy controls.
CAD is the most common cardiovascular disease, with high morbidity and mortality, serious threat to human health, and coronary atherosclerosis is its pathogenesis. It is generally believed that endothelial dysfunction, vascular inflammation and lipid metabolism disorder are the more important cause of CAD (15). Vascular endothelial cells are a barrier between the blood and the blood vessel wall can produce endothelin, nitric oxide, prevent thrombosis and platelet adhesion (16). The damage of vascular endothelial cells is a key factor in the early stage of atherosclerosis, and is also an important link in the early stage of coronary atherosclerosis and acute coronary syndrome (17). Inflammatory factors play an important role in the process of coronary atherosclerosis. Studies have shown that IL-6, C-reactive protein, interleukin 10 and other inflammatory cytokines expression in patients with CAD was significantly increased, and closely related to the severity of the disease (18-20).
Adropin is a newly discovered endogenous bioactive substance that is mainly expressed in the heart, brain, liver and coronary endothelial cells (21). Adropin is involved in regulating lipid metabolism, improving insulin resistance, protecting vascular endothelial cells, and anti-inflammatory effects (4-6). Adropin can increase the endothelial nitric oxide synthase expression, which has a certain endothelial cell protection potential (22). Circulating low levels of adropin are associated with endothelial dysfunction (23); decreased serum adropin levels weaken endothelial protection and may cause or accelerate atherosclerosis. Therefore, consider the level of adropin and CAD may have a certain relationship. In the 33 CAD patients and 30 healthy controls, serum adropin was detected by ELISA. The level of serum adropin in CAD group (1.91 ng/mL) was lower than that in healthy controls (3.19 ng/mL) (10). Yu et al. also found that serum adropin levels in CAD patients were lower than those of healthy controls (13). However, due to the small number of patients involved in the current literature, the quality of the literature is not high. In this study, meta-analysis was used to evaluate the relationship between serum adropin level and CAD in a comprehensive and systematic way, further demonstrating that serum adropin levels in CAD patients were lower than those in healthy controls. This study brings together outstanding papers published by core journals both at home and abroad, screened the selected documents strictly, selected more articles, large sample size and high credibility.
This meta-analysis also has some limitations: (I) the quality of meta-analysis is based on the quality of the research, and the quality of this meta-analysis is relatively general; (II) the number of included studies is relatively inadequate and the sample size is relatively small, so the statistical power of this meta-analysis is relatively inadequate; (III) meta-analysis is based on a summary of past research literature, and may expand the sample size and improve the credibility of the conclusion, but also may accumulate the bias of the corresponding original document.
Conclusions
In summary, serum adropin levels in patients with CAD were significantly lower, suggesting that serum Adropin levels may be associated with the pathogenesis of CAD. However, due to the limited number of included studies and the small number of cases, the above conclusion still needs more high-quality research to be verified.
Acknowledgements
Funding: The study was supported by the Scientific Research Development Program of North Sichuan Medical College (Key Program: CBY16-A-ZD10).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mann D, Zipes D, Libby P, et al. Braunwald’s heart disease: a textbook of cardiovascular medicine, single volume. 10th ed. Philadelphia, PA: Elsevier Health Sciences Division, 2012.
- Zhang C, Zhao L, Xu W, et al. Correlation of serum adropin level with coronary artery disease. Zhonghua Yi Xue Za Zhi 2014;94:1255-7. [PubMed]
- Kumar KG, Trevaskis JL, Lam DD, et al. Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab 2008;8:468-81. [Crossref] [PubMed]
- Gao S, McMillan RP, Jacas J, et al. Regulation of substrate oxidation preferences in muscle by the peptide hormone adropin. Diabetes 2014;63:3242-52. [Crossref] [PubMed]
- Ganesh Kumar K, Zhang J, Gao S, et al. Adropin deficiency is associated with increased adiposity and insulin resistance. Obesity (Silver Spring) 2012;20:1394-402. [Crossref] [PubMed]
- Aydin S, Kuloglu T, Aydin S, et al. Expression of adropin in rat brain, cerebellum, kidneys, heart, liver, and pancreas in streptozotocin-induced diabetes. Mol Cell Biochem 2013;380:73-81. [Crossref] [PubMed]
- Topuz M, Celik A, Aslantas T, et al. Plasma adropin levels predict endothelial dysfunction like flow-mediated dilatation in patients with type 2 diabetes mellitus. J Investig Med 2013;61:1161-4. [Crossref] [PubMed]
- Du HC, Wang JH, Du WP, et al. Clinical significance of serum adropin protein in the diagnosis and treatment of coronary heart disease patients with angina pectoris. Hebei Yi Yao Za Zhi 2015;37:3096-9.
- Ertem AG, Ünal S, Efe TH, et al. Association between serum adropin level and burden of coronary artery disease in patients with non-ST elevation myocardial infarction. Anatol J Cardiol 2017;17:119-24. [PubMed]
- Mu Z, Wu ZH, Yan SC, et al. Changes of serum adropin in patients with acute coronary syndrome. J Chin-Jap Frie Hosp 2014;28:273-6.
- Qi R, Li K. Correlation between serum adropin and TNF-α in patients with acute myocardial infarction. J Hubei Univ Sci Tech 2015;29:468-9.
- Xu X, Yang XL. The effects of adropin and pentaprotein-3 on acute coronary syndrome. Chin Commu Doc 2016;32:148-9.
- Yu HY, Zhao P, Wu MC, et al. Serum adropin levels are decreased in patients with acute myocardial infarction. Regul Pept 2014;190-191:46-9. [Crossref] [PubMed]
- Zhao LP, Xu WT, Wang L, et al. Serum adropin level in patients with stable coronary artery disease. Heart Lung Circ 2015;24:975-9. [Crossref] [PubMed]
- Yu XH, Qian K, Jiang N, et al. ABCG5/ABCG8 in cholesterol excretion and atherosclerosis. Clin Chim Acta 2014;428:82-8. [Crossref] [PubMed]
- Navab KD, Elboudwarej O, Gharif M, et al. Chronic inflammatory disorders and accelerated atherosclerosis: chronic kidney disease. Curr Pharm Des 2011;17:17-20. [Crossref] [PubMed]
- Yoon MH, Reriani M, Mario G, et al. Long-term endothelin receptor antagonism attenuates coronary plaque progression in patients with early atherosclerosis. Int J Cardiol 2013;168:1316-21. [Crossref] [PubMed]
- Wang XH, Liu SQ, Wang YL, et al. Correlation of serum high-sensitivity C-reactive protein and interleukin-6 in patients with acute coronary syndrome. Genet Mol Res 2014;13:4260-6. [Crossref] [PubMed]
- Liu J, Jia Y, Li X, et al. Serum interleukin-10 levels and adverse events in patients with acute coronary syndrome: a systematic review and meta-analysis. Chin Med J (Engl) 2014;127:150-6. [Crossref] [PubMed]
- Al Shahi H, Shimada K, Miyauchi K, et al. Elevated Circulating Levels of Inflammatory Markers in Patients with Acute Coronary Syndrome. Int J Vasc Med 2015;2015:805375. [Crossref] [PubMed]
- Petersen TN, Brunak S, von Heijne G, et al. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 2011;8:785-6. [Crossref] [PubMed]
- Lovren F, Pan Y, Quan A, et al. Adropin is a novel regulator of endothelial function. Circulation 2010;122:S185-92. [Crossref] [PubMed]
- Oruc CU, Akpinar YE, Dervisoglu E, et al. Low concentrations of adropin are associated with endothelial dysfunction as assessed by flow-mediated dilatation in patients with metabolic syndrome. Clin Chem Lab Med 2017;55:139-44. [Crossref] [PubMed]