Cardiac magnetic resonance imaging myocardial perfusion reserve index assessment in women with microvascular coronary dysfunction and reference controls
Introduction Other Section
Women with signs or symptoms of myocardial ischemia but no obstructive coronary artery disease (CAD) frequently have microvascular coronary dysfunction (MCD). The diagnosis of MCD is based on endothelial and non-endothelial dependent function determined using invasive coronary reactivity testing (CRT). Patients with MCD have reduced coronary flow reserve and metabolic evidence of ischemia (1). In the absence of CAD, MCD can occur due to iatrogenic dysfunction or as a counterpart to traditional coronary risk factors (2). To date, CRT remains the gold standard test for MCD which is an invasive, highly technical test that requires advanced trained interventional cardiologists and can be time consuming. Prior work suggests that adenosine cardiac magnetic resonance imaging (CMRI) may be a useful non-invasive method for detection of MCD (3-5). Global magnetic resonance perfusion imaging, together with ejection fraction, has been found to predict prognosis in women with signs and symptoms of ischemia but no obstructive CAD (6). The diagnosis of MCD carries adverse prognoses including increased rates of cardiovascular death, non-fatal myocardial infarction, stroke, and heart failure (7-10).
Adenosine CMRI is a non-invasive imaging technique that may be useful for the detection of perfusion abnormalities consistent with MCD by comparison of rest to vasodilator stress myocardial perfusion. Myocardial perfusion reserve determined by CMRI correlates with invasive coronary flow reserve by Doppler flow wire (11), and CMRI has been found to be highly sensitive for evaluating coronary blood flow (12,13). We evaluated CMRI in patients diagnosed with MCD by CRT (cases) and reference controls using semi-quantitative CMRI myocardial perfusion reserve index (MPRI) analysis.
Methods Other Section
Subjects
Fifty-three women with signs and symptoms of ischemic heart disease, ischemia on cardiac stress testing and no obstructive CAD underwent clinically indicated CRT for suspected MCD. Subsequently, all MCD cases and 12 asymptomatic reference controls who were age-, and estrogen-use matched to the case subjects underwent CMRI. Reference controls qualified for participation by performing a maximal exercise Bruce protocol treadmill test that was within normal limits and had no traditional cardiac risk factors. All women were recruited from the Women’s Heart Center at the Cedars-Sinai Heart Institute in Los Angeles, California. This study was approved by the Cedars-Sinai internal review board and written informed consent was obtained for all subjects.
MCD was defined as endothelial- or non-endothelial-dependent vascular function abnormality found by CRT as previously published (9,14). Briefly, all women with signs or symptoms of ischemia underwent coronary angiography; Those with no obstructive CAD (defined as epicardial coronary artery stenosis of <20%) underwent CRT with intracoronary adenosine, acetylcholine, and nitroglycerine to determine endothelial and non-endothelial, micro- and macro-vascular function, as previously published (14).
CMRI protocol
CMRI was performed on all subjects in the supine position on a 1.5-Tesla CMRI scanner (Siemens Sonata, Erlangen, Germany) with electrocardiogram (ECG)-gating and a phased-array surface coil (CP Body Array Flex, Siemens Medical Systems, Erlangen, Germany) using a previously published highly standardized protocol that included assessment of cardiac function and viability in addition to adenosine and rest perfusion imaging and a total dose of gadolinium contrast of 0.15 mmol/kg (Gadodiamide, Omniscan, Amersham, Piscataway, NJ, USA) (Figure 1) (3,4). Blood pressure and pulse oxygenation were monitored (In vivo, Philadelphia, PA, USA) and recorded before, during and after adenosine infusion. A 12 lead ECG was recorded prior to and following CMRI.
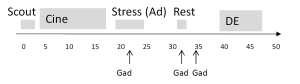
The left ventricular short axis was determined by scout imaging, and perfusion images were obtained in basal, mid and distal short axis image planes. A GRE-Epi hybrid pulse sequence was used for all patients: field of view 350 mm × 350 mm minimized dependent upon patient size, slice thickness 8 mm, TE/TR maximum 1.33/6.5, bandwidth 1,420 Hz, GRAPPA acceleration factor 2, imaging every heartbeat. Pharmacologic adenosine vasodilation was used in all subjects (Adenoscan, Astellas Pharma US, Inc) at a dose of 140 mcg/kg/min IV over a total duration of 4 minutes with mid adenosine injection of 0.05 mmol/kg gadolinium at 4 mL/s via a second IV catheter, followed by 30 mL saline at 4 mL/s. This was followed 10 minutes later by rest perfusion imaging with the same contrast infusion settings.
Quantitative analysis MPRI
Quantitative analysis of the first pass perfusion images for MPRI was performed using CAAS MRV 3.3 software (Pie Medical Imaging B.V., Netherlands) by skilled users (LT, PG). Epicardial and endocardial left ventricle (LV) myocardial contours (basal, midventricular, and apical slices) were manually determined in order to acquire intensity over time curves at rest and adenosine for 16-segments (segment 17, the LV apex, was not acquired). The relative upslope (RU) was defined as the maximum upslope of the selected curve, divided by the maximum upslope of the left ventricular cavity curve. Subsequently, RU was used for calculation of the MPRI, defined as the ratio of RU (adenosine)/RU (rest) for each segment and for global, subendocardial and subepicardial regions (Figure 2).
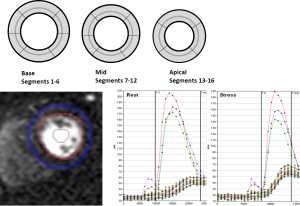
Global area was defined as the mean of segments 1-16. Within each segment the outer 50% region was defined as subepicardium, and the inner 50% region was defined as the subendocardium. Segmental areas were defined as basal, the mean of segments 1-6, midventricular, the mean of segments 7-12 and apical, the mean of segments 13-16. Vascular territories were defined as left anterior descending artery as the mean of segments 1, 2, 7, 8, 9, 13, and 14; left circumflex artery as the mean of segments 5, 6, 11, 12, 16; and right coronary artery mean of segments 3, 4, 10, and 15.
Statistical analysis
All statistical analysis was performed using SAS (ver. 9.2; The SAS Institute, Cary, NC). Summary data are expressed as means standard deviation (SD) for continuous variables and frequencies (%) for categorical ones. Since all the continuous variables are approximately normally distributed, student t test was used to test the differences between groups. Fisher’s exact test compared categorical data due to low frequencies. Comparisons of subendocardial vs. subepicardial MPRI within the study group were conducted using paired t-test. Repeated measures ANOVA models were used to test the differences between the left circumflex artery, left anterior descending and right coronary artery and between segmental regions for either the MCD cases or normal controls while taking into account data correlation. Statistical significance was considered P<0.05.
Results Other Section
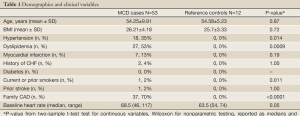
Table 1 outlines the baseline characteristics of the MCD cases and reference controls. Over half of MCD patients reported daily chest pain. MCD was diagnosed by CRT and the median left ventricular end diastolic filling pressure was 15 mmHg (range, 5.0-24.0 mmHg) and median coronary flow reserve to intracoronary adenosine was 2.60 (range, 1.5-3.8). Median coronary blood flow was 21.5 (range, –81.0-242.5), and median percent constriction was –9.3% (range, –65.5-38.8%) in response to intracoronary acetylcholine. Smooth muscle response to intracoronary nitroglycerin was vasodilation by 16.4% (range, –14.1-52.4%).
Full Table
All subjects completed the CMRI protocol and there were no adverse events during testing. All data was interpretable in 16 segments for pharmacologic adenosine and rest perfusion.
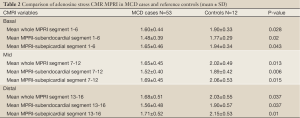
There was a significant difference in MPRI between MCD cases and reference controls in terms of both global and segmental values. Overall, globally MCD cases had lower MPRI than the reference controls (Table 2). This difference was also observed in both subendocardial and subepicardial sub segment analysis (Figure 3).
Full Table
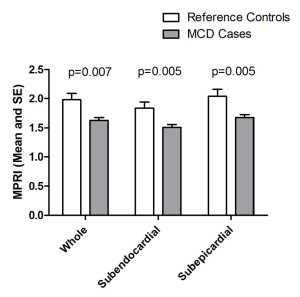
Within the study population, subendocardial MPRI was lower than subepicardial MPRI. In the MCD cases, subendocardial vs. subepicardial MPRI was 1.51±0.35 vs. 1.68±0.38 (P<0.0001) and in the reference control this was 1.84±0.34 vs. 2.04±0.41 (P=0.001). This transmural gradient between subendocardial and subepicardial was observed in all 3 slices (apical, midventricular and basal) in both the MCD cases and reference controls (data not shown, all P<0.01). There was no difference in the subendocardial to subepicardial MPRI ratio for MCD subjects vs. reference controls (midventricular MPRI 0.90±0.10 vs. 0.93±0.10, P=0.46).
Within the vascular territories, both the MCD cases and the reference controls had lower MPRI in the left circumflex artery when compared to the left anterior descending and right coronary artery. There were no differences however, in MPRI between basal, midventricular and apical slices for either the MCD cases or reference controls (Table 3).

Full Table
Discussion Other Section
The current study results demonstrate that women with MCD have a significantly lower MPRI when compared to matched reference controls. This difference is global, being observed in all myocardial territories, and not localized to an epicardial coronary artery distribution. Our results support the hypothesis that semi-quantitative CMRI MPRI is diffusely reduced in women who at CRT have abnormal coronary flow reserve secondary to MCD (15-18).
Our results are consistent with much of the prior literature. Prior study has demonstrated that CMRI more accurately detects impaired coronary flow than quantitative coronary angiography in patients with non-severe coronary stenosis (15). Panting et al. first studied a cohort of men and women with cardiac syndrome X (CSX) compared to matched controls using CMRI and found a significantly different perfusion response to vasodilator in the CSX cases when considering the ratio of subendocardial vs. subepicardial indexed myocardial perfusion reserve (5). In addition, perfusion differences across the myocardial wall segments were previously reported in patients with CSX (19). Unlike Panting et al., we found a difference in transmural, subendocardial and subepicardial MPRI between MCD cases and reference controls. The ratio of subendocardial vs. subepicardial MPRI was not different between groups.
Our current study results are not consistent with a recent study that reported no difference in absolute quantitative and visual 3T CMRI perfusion and blood oxygen level-dependent oxygenation in 18 CSX cases compared to 14 reference controls (20). These investigators used a very computationally intensive approach to measure rest and Adenosine stress coronary flow which was additionally normalized by the rate pressure product; thus a different measure of coronary flow reserve than MPRI. Another factor that limits comparability to this study is that the CSX cases did not have invasive confirmation of MCD, and may therefore have been a more heterogeneous or lower risk population.
In women with chest pain and open coronary arteries, a stress-induced reduction in the myocardial phosphocreatine-ATP ratio measured by magnetic resonance spectroscopy (MRS) is consistent with myocardial ischemia. Such abnormal MRS also predicts adverse CVD outcomes (8). The mechanism of myocardial ischemia in women with MCD is believed to be due to the inability of the microvasculature to match the increased myocardial blood flow demand, similar to what is seen with epicardial stenosis (1). MCD therefore should result in diffuse perfusion abnormalities rather than segmental abnormalities seen with obstructive CAD. One potential pathologic mechanisms of myocardial infarction in the setting of open coronary arteries is endothelial dysfunction with the inability to increase coronary flow in response to stress (6).
Our results extend the prior literature to indicate that the perfusion reserve pattern observed in MCD patients is global, diffuse and circumferential, e.g., not restricted to one vascular territory. Although we found a lower MPRI in the left circumflex artery distribution in both the MCD case and reference controls it is unclear whether this observed difference is a function of imaging technique, such as lower signal observed in those segments, or represents true variation in MPRI. This observed difference is a subject for further research.
Strengths of our study include a well characterized cohort of women with MCD diagnosed by invasive CRT and careful selection of age and estrogen status matched reference controls. The requirement of the reference control subjects to have a reference exercise response to high workload Bruce protocol treadmill testing was to ensure an appropriately healthy control group. We used standardized protocols for the exercise and adenosine pharmacological testing, as well as CMRI image acquisition and quantitative analysis of MPRI. While a limitation to our current study is the relatively small number of cases and controls, our dataset is larger than most literature to date. A second limitation may have been the lack of patients with diabetes in this study population possibly due to a selection bias as the inclusion criteria were women with <20% obstructive disease with persistent chest pain. It is likely that we would have observed a greater difference between MCD cases and reference controls if the population had included patients with diabetes. We hypothesize that patients with diabetes are being treated for MCD without undergoing invasive testing. Prospective validation of a diagnostic threshold for MPRI in patients with MCD is ongoing in our NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (21).
In conclusion, CMRI MPRI is lower in MCD cases as compared to reference controls and appears to be global and not segmental. This group difference occurs in both the subendocardial and subepicardial segments. While invasive CRT remains the gold standard for the diagnosis of MCD, our findings suggest that non-invasive adenosine pharmacological CMRI MPRI may be a useful noninvasive tool in the evaluation of patients with MCD.
AcknowledgementsOther Section
This work was supported by contracts from the National Heart, Lung and Blood Institutes, nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, grants U0164829, U01 HL649141, U01 HL649241, T32HL69751, 5R01HL090957, 1R03AG032631 from the National Institute on Aging, GCRC grant MO1-RR00425 from the National Center for Research Resources and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ, the UF Clinical and Translational Science Award NIH/NHLBI 5UL1RR025208-02, The Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, CA, the Edythe L. Broad Women’s Heart Research Fellowship, Cedars-Sinai Medical Center, Los Angeles, California, and the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles.
Disclosure: Dr. Berman discloses honorarium from Astellas Pharma and Dr. Bruce Samuels discloses speaker’s bureau for Abbott Vascular and Volcano Corporation. No other authors have conflicts of interests.
ReferencesOther Section
- Herrmann J, Kaski JC, Lerman A. Coronary microvascular dysfunction in the clinical setting: from mystery to reality. Eur Heart J 2012;33:2771-2782b. [PubMed]
- Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med 2007;356:830-40. [PubMed]
- Ishimori ML, Martin R, Berman DS, et al. Myocardial ischemia in the absence of obstructive coronary artery disease in systemic lupus erythematosus. JACC Cardiovasc Imaging 2011;4:27-33. [PubMed]
- Mehta PK, Goykhman P, Thomson LE, et al. Ranolazine improves angina in women with evidence of myocardial ischemia but no obstructive coronary artery disease. JACC Cardiovasc Imaging 2011;4:514-22. [PubMed]
- Panting JR, Gatehouse PD, Yang GZ, et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med 2002;346:1948-53. [PubMed]
- Doyle M, Weinberg N, Pohost GM, et al. Prognostic value of global MR myocardial perfusion imaging in women with suspected myocardial ischemia and no obstructive coronary disease: results from the NHLBI-sponsored WISE (Women’s Ischemia Syndrome Evaluation) study. JACC Cardiovasc Imaging 2010;3:1030-6. [PubMed]
- Gulati M, Cooper-DeHoff RM, McClure C, et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med 2009;169:843-50. [PubMed]
- Johnson BD, Shaw LJ, Buchthal SD, et al. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation 2004;109:2993-9. [PubMed]
- von Mering GO, Arant CB, Wessel TR, et al. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation 2004;109:722-5. [PubMed]
- Bugiardini R, Bairey Merz CN. Angina with “normal” coronary arteries: a changing philosophy. JAMA 2005;293:477-84. [PubMed]
- Kurita T, Sakuma H, Onishi K, et al. Regional myocardial perfusion reserve determined using myocardial perfusion magnetic resonance imaging showed a direct correlation with coronary flow velocity reserve by Doppler flow wire. Eur Heart J 2009;30:444-52. [PubMed]
- Sakuma H, Higgins CB. Magnetic resonance measurement of coronary blood flow. Acta Paediatr Suppl 2004;93:80-5. [PubMed]
- Sakuma H, Kawada N, Takeda K, et al. MR measurement of coronary blood flow. J Magn Reson Imaging 1999;10:728-33. [PubMed]
- Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol 2010;55:2825-32. [PubMed]
- Barmeyer AA, Stork A, Muellerleile K, et al. Comparison of quantitative coronary angiography and first-pass perfusion magnetic resonance imaging for the detection of an impaired coronary perfusion in nonsevere coronary stenosis. J Magn Reson Imaging 2008;27:1005-11. [PubMed]
- Lanza GA, Buffon A, Sestito A, et al. Relation between stress-induced myocardial perfusion defects on cardiovascular magnetic resonance and coronary microvascular dysfunction in patients with cardiac syndrome X. J Am Coll Cardiol 2008;51:466-72. [PubMed]
- Wöhrle J, Nusser T, Merkle N, et al. Myocardial perfusion reserve in cardiovascular magnetic resonance: correlation to coronary microvascular dysfunction. J Cardiovasc Magn Reson 2006;8:781-7. [PubMed]
- Yilmaz A, Athanasiadis A, Mahrholdt H, et al. Diagnostic value of perfusion cardiovascular magnetic resonance in patients with angina pectoris but normal coronary angiograms assessed by intracoronary acetylcholine testing. Heart 2010;96:372-9. [PubMed]
- Vermeltfoort IA, Bondarenko O, Raijmakers PG, et al. Is subendocardial ischaemia present in patients with chest pain and normal coronary angiograms? A cardiovascular MR study. Eur Heart J 2007;28:1554-8. [PubMed]
- Karamitsos TD, Arnold JR, Pegg TJ, et al. Patients with syndrome X have normal transmural myocardial perfusion and oxygenation: a 3-T cardiovascular magnetic resonance imaging study. Circ Cardiovasc Imaging 2012;5:194-200. [PubMed]
- Shufelt CL, Johnson DB, Mehta PK, et al. Noninvasive Detection of microvascular coronary dysfunction using cardiac magnetic resonance imaging: the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study. Circulation 2011;124:A1036.



