
Total Astragalus saponins attenuates CVB3-induced viral myocarditis through inhibiting expression of tumor necrosis factor α and Fas ligand
Introduction
Astragalus L. is the largest genus in the family Leguminosae. It is a very old and well-known drug traditional medicine used as an antiperspirant, tonic and diuretic (1). Several in vivo experiments have illustrated the cardiovascular-protective effects of Astragalus L., which may be due (at least in part) to its anti-oxidative (2), anti-inflammatory and anti-apoptotic activities and endothelium-protective role under pathophysiologic conditions (3).
Total Astragalus saponins (AST) exhibit anti-inflammatory activities and can induce the apoptosis and growth inhibition of tumor cells. It has been reported that AST can influence the transforming growth factor beta-1/suppressor of mothers against decapentaplegic, mammalian target of rapamycin, extracellular signal-regulated kinase (ERK) and ERK-independent nuclear factor-kappa B signaling pathways (3). However, the biologic mechanism of AST is not known.
Viral myocarditis (VMC) is a major factor in dilated cardiomyopathy (DCM) and sudden death in young people. An abnormal immune reaction and cytokine expression have crucial roles in VMC development (4). The primary characteristic of early-stage VMC in vivo is excessive proliferation of lymphoid cells and increased conversion of metrocytes. Expression of monocyte chemotactic protein-1 in VMC patients promotes macrophage infiltration into the myocardium. The increase in the number of myocardial macrophages can lead to high levels of nitric oxide as well as expression of interleukin (IL)-12, IL-1, and tumor necrosis factor (TNF)-α, which increases the likelihood of VMC (4).
The pathogenesis of VMC is not clear, and a specific clinical treatment is lacking. Antiviral agents are used mainly in the early stages of VMC. In the middle and late stages of VMC, immunosuppressant agents are chosen to reduce the myocardial damage induced by an abnormal immune response and symptomatic treatments (5).
We wished to evaluate the therapeutic effect of AST on coxsackie B (CVB) 3 -induced myocarditis in vivo and in vitro. We hoped that our data could provide a theoretical basis for VMC treatment.
Methods
Ethical approval of the study protocol
All experiments were approved by the Animal Care and Use Committee of Inner Mongolia Medical University (Hohhot, Inner Mongolia) and were in accordance with Guide for Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA).
Reagent
AST (purity =99%) was obtained from Huadong Medicines (Hangzhou, China). A Cell Counting Kit-8 (CCK-8) kit was purchased from Sigma-Aldrich (Saint Louis, MO, USA). RPMI-1640 medium was from Thermo Fisher Scientific (Waltham, MA, USA). All other reagents were of certified analytical reagent grade.
Animals, virus injection and treatment
BALB/C mice (18–22 g) were purchased from the Animal Center of Inner Mongolia University. Mice were fed a standard laboratory diet and water, and kept at 24±1 °C and a relative humidity of 50–60% with a 12-h light-dark cycle.
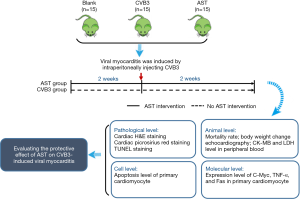
BALB/C mice was randomly divided into three groups, namely blank group (n=15), VMC group (CVB3, n=15) and AST treated group (AST, n=15). Before CVB3 injection, AST-IV treated mice received 100 mg/kg of AST (3 mL/30 g bw) per day by gavage to performed a pretreatment. Blank and CVB3-induced mice received 300 µL of saline orally per day. After pretreatment for 2 weeks, mice (CVB3-induced mice and AST-IV treated mice) were induced VMC by an intraperitoneal injection with 0.1 mL of PBS containing 103 50% tissue culture infective dose (TCID50) CVB3. After virus was injected, AST-IV treated mice continuously received 100 mg/kg of AST-IV (3 mL/30 g bw) per day by gavage until the end of experiment (Figure 1).
Enzyme-linked immunosorbent assays (ELISAs) for lactate dehydrogenase (LDH) and creatine kinase-MB (CK-MB)
The levels of LDH and CK-MB in the peripheral blood and heart-tissue homogenates of mice were determined by ELISA following manufacturer (eBioscience, San Diego, CA, USA) instructions.
Histology
Mouse hearts were fixed in 4% paraformaldehyde, embedded in paraffin, and cut transversely into sections of thickness 5 µm. Serial heart sections were stained with hematoxylin and eosin (H&E) to measure hypertrophic growth. The degree of collagen deposition was detected by Masson staining. Images were analyzed using a 5/25 quantitative digital image-analysis system (Image-Pro® Plus 6.0).
Cell culture, viral infection and drug treatment
CVB3 (Nancy strain) was maintained by passage through HeLa cells. Virus titers were determined through a TCID50 assay of HeLa cells and calculated by the Reed-Muench method (6). Briefly, we inoculated 2×106 cells/mL primary myocardiocytes (PMCs) into 96 wells (0.1 mL for each well) and cultivated them for 1–2 days to form a PMC monolayer. PMCs were infected with CVB3 (1,000 TCID50 per well) after treatment with AST (100 µM) for 24 h. We observed the progress of cytopathic effects (CPEs) with a microscope, and recorded the results each day. Cultivation was terminated when nearly all PMCs in the virus control group had disintegrated. PMCs were stained according to the instructions of the MTT staining kit and the absorbance was read at 490 nm.
Apoptosis assay for PMCs
PMCs were stained using a fluorescein isothiocyanate (FITC) annexin V apoptosis detection kit (Becton Dickinson, Franklin Lake, NJ, USA) and then measured by flow cytometry (C6 flow cytometer; Becton Dickinson) to measure PMC apoptosis after infection and treatment. Data were analyzed using FlowJo v7.6 (FlowJo LLC, Ashland, OR, USA).
Real-time polymerase chain reaction (PCR) detection of the mRNA levels of C-Myc, TNF-á and Fas
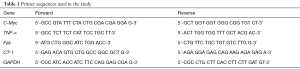
Total cellular RNA was extracted by standard methods with TRIzol® (Life Technologies, Carlsbad, CA, USA) after PMCs had been infected and treated. mRNA was synthesized using first-strand cDNA by a reverse transcription kit (Takara Biotechnology, Shiga, Japan). The primer sequences used in the experiments are shown in Table 1.
Full table
Statistical analyses
Data are the mean ± standard error of the mean (SEM). Differences between experimental groups were analyzed for significance using one-way ANOVA in Prism v5.0 (GraphPad, San Diego, CA, USA). P<0.05 was considered significant.
Results
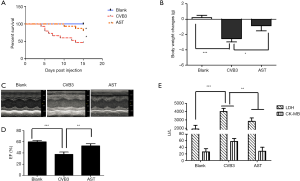
AST treatment attenuates CVB3-induced myocarditis in mice
To investigate the effect of AST on CVB3-induced myocarditis, mice were first treated with 10 mg/kg AST for 2 weeks and then infected with CVB3 or given PBS.
As expected, mice receiving PBS alone developed no sign of VMC, whereas the signs of VMC were apparent in CVB3-infected mice, including weakness and weight loss (Figure 2A), with 53% of mice dying 15 days after infection (Figure 2B). In contrast, AST administration ameliorated CVB3-induced myocarditis significantly in the AST group: ~80% of mice survived without suffering severe VMC (Figure 2B) in the AST (10 mg/kg) group (P<0.05) and was accompanied by slight loss in body weight (Figure 2A). The ejection fraction of mouse hearts in the CVB3 group was significantly lower (P<0.01) compared with that in the blank group, and AST administration could significantly increase the ejection fraction (P<0.05, Figure 2C,D). Compared with blank groups, levels of LDH and CM-KB in heart-tissue homogenates were higher in the CVB3 group, whereas their levels in the AST group were decreased significantly (P<0.01, Figure 2E). Taken together, AST treatment seemed to attenuate CVB3-induced myocarditis in mice.
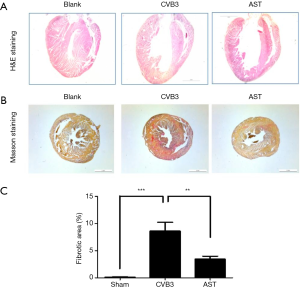
AST treatment attenuates CVB3-induced myocardial dilation and fibrosis
To investigate the potential effect of AST on CVB3-induced myocardial dilation and fibrosis in vivo, cross-sections of heart tissue were colored with H&E and Masson stains. As expected, AST inhibited CVB3-induced myocardial dilation significantly (Figure 3A). In CVB3-induced myocardial dilation, Mason dyeing results showed that the level of fibrosis in heart tissue was increased significantly. However, compared with the CVB3 group, AST could attenuate the degree of collagen deposition significantly (P<0.01) (Figure 3B,C). Consistent with these data, AST treatment attenuated CVB3-induced dilation and fibrosis within the myocardium.
AST treatment attenuates CVB3-induced changes in the viability and apoptosis of PMCs in vitro and in vivo
First, the CCK-8 kit was used to assess the effect of AST on CVB3-induced changes in PMC viability. AST could inhibit the CVB3-induced decrease in PMC viability significantly (Figure 4A).
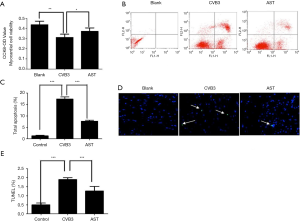
Based on our results, PMCs and heart-tissue sections were respectively stained with FITC annexin V and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL). As expected, AST could inhibit CVB3-induced PMC apoptosis significantly (Figure 4B,C). Compared with the blank group, the mean apoptosis percentage in the CVB3 group was 17.35%, whereas that in the AST group was 7.64% (P<0.05). TUNEL staining of heart-tissue sections revealed that CVB3 could induce PMC apoptosis in vivo, and that the apoptosis percentage was attenuated significantly in the AST group (Figure 4D,E; P<0.05). Consistent with these data, AST could inhibit changes in CVB3-induced PMC viability, which was associated with inhibition of CVB3-induced PMC apoptosis.
Effect of AST on CVB3-induced increased expression of C-Myc, TNF-á and Fas
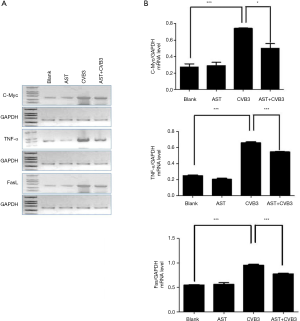
CVB3-infected PMCs were treated with AST, mRNA was extracted by TRIzol, and mRNA was amplified by specific primers after synthesis of first-strand cDNA by reverse transcription. Compared with the blank group, CVB3 could increase the mRNA level of C-Myc, TNF-α and Fas significantly. These moieties have a close relationship with the apoptosis, inflammation and necrosis of myocardial cells in patients suffering from VMC. AST treatment could reduce the mRNA level of C-Myc, TNF-α and Fas significantly (P<0.01, Figure 5A,B), which may be associated with the therapeutic effect of AST on CVB3-induced myocarditis in mice.
Discussion
We showed the therapeutic effect of AST on CVB3-induced myocarditis. Our study elicited three main findings. First, AST reduced CVB3-induced pathologic changes in myocardial tissue in vivo, including myocardial hypertrophy and fibrosis. Second, AST had a protective effect on the CVB3-induced CPEs of PMCs in vitro. Third, AST could significantly inhibit the increase in CVB3-induced transcription of C-Myc, TNF-α and Fas, which have a close relationship with the apoptosis, inflammation and necrosis of myocardial cells.
The CPEs in the early phase of CVB3-induced myocarditis has been attributed mainly to apoptosis and viral replication (7). Studies have shown that mitochondrial fission as well as phosphatidylinositol-4,5-bisphosphate 3-kinase and Fas ligand (FasL)/Fas signaling pathways are involved in CVB3-induced myocardial apoptosis (8,9). The interaction between FasL and Fas recruits an adapter protein with a death domain, which in turn employs its death effector domain to induce expression of procaspase-8 and -10 (10,11).
The present study showed that caspase-8 could cleave downstream of caspase-3 activation. This phenomenon is partially or totally responsible for the proteolytic cleavage of many death substrates, such as the nuclear enzyme poly (ADP-ribose) polymerase, which leads to myocardial apoptosis through a capase-3-dependent signaling pathway (12,13). We found that AST could significantly inhibit CVB3-induced expression of FasL and PMC apoptosis in vivo and in vitro, which may contribute to the protective effect of AST on fibrosis and dilation within the myocardium.
c-Myc is a member of the Myc family of b/HLH/LZ proteins that regulate the proliferation and apoptosis of cells (14). Although its exact function remains unclear, the Myc family appears to be necessary for CVB3-induced myocardial apoptosis (15). The present study showed that a mitochondrial-related apoptotic pathway was associated with c-Myc (16). Also, c-Myc expression is very high in neonatal mouse hearts for the promotion of myocardial-cell proliferation and heart development. Several studies have shown that some genes, especially those associated with heart development, are overexpressed in mature hearts suffering from dilated or hypertrophy cardiomyopathy (17). In our study, compared with the CVB3 group, c-Myc expression was very low in the AST group, which may have been associated with the protective role of AST on CVB3-induced DCM and PMC apoptosis.
The end result of VMC is DCM, especially in the young. The protective mechanism of AST on CVB3-induced myocarditis could be related to a reduction in the endogenous level of TNF-α. Increasing the TNF-α level plays an important part in the pathologic changes of congestive heart failure (18). Yu and colleagues showed that TNF-α-secreting B-cells could promote proliferation of cardiac fibroblasts and regulate collagen production through ERK1/2 signaling in vitro (18). Das and coworkers showed that TNF-α could promote the apoptosis of myocardial cells through activation of p38 mitogen-activated protein kinase and deactivation of ERK1/2 pathways, which may contribute towards the development of cardiac dysfunction in DCM (19). In our study, AST could significantly decrease TNF-α secretion of PMCs, which would have an important role in slowing the progress of CVB3-induced myocarditis.
Conclusions
This study demonstrates that AST has a therapeutic effect on CVB3-induced myocarditis. This effect was associated with attenuating CVB3-induced myocardial dilation, apoptosis and fibrosis through inhibiting expression of FasL, c-Myc and TNF-α.
Acknowledgments
We would like to thank the native English-speaking scientists of Elixigen Company (Huntington Beach, California) for editing our manuscript.
Funding: This work was supported by the National Natural Science Foundation of China (grant number 81460066).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All experiments were approved by the Animal Care and Use Committee of Inner Mongolia Medical University (Hohhot, Inner Mongolia) (No. YKD2015065) and were in accordance with Guide for Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA).
References
- Polat E, Bedir E, Perrone A, et al. Triterpenoid saponins from Astragalus wiedemannianus Fischer. Phytochemistry 2010;71:658-62. [Crossref] [PubMed]
- Huang YF, Lu L, Zhu DJ, et al. Effects of Astragalus Polysaccharides on Dysfunction of Mitochondrial Dynamics Induced by Oxidative Stress. Oxid Med Cell Longev 2016;2016:9573291. [Crossref] [PubMed]
- Abdullahi AY, Kallon S, Yu X, et al. Vaccination with Astragalus and Ginseng Polysaccharides Improves Immune Response of Chickens against H5N1 Avian Influenza Virus. Biomed Res Int 2016;2016:1510264. [Crossref] [PubMed]
- Patmanathan SN, Yap LF, Murray PG, et al. The antineoplastic properties of FTY720: evidence for the repurposing of fingolimod. J Cell Mol Med 2015;19:2329-40. [Crossref] [PubMed]
- Snyder M. Pediatric viral myocarditis. Air Med J 2003;22:6-8. [Crossref] [PubMed]
- Wang C, Dong C, Xiong S. IL-33 enhances macrophage M2 polarization and protects mice from CVB3-induced viral myocarditis. J Mol Cell Cardiol 2017;103:22-30. [Crossref] [PubMed]
- Li X, Zhang J, Chen Z, et al. Both PI3K- and mTOR-signaling pathways take part in CVB3-induced apoptosis of Hela cells. DNA Cell Biol 2013;32:359-70. [Crossref] [PubMed]
- Chang H, Han B, Han XZ. The mechanisms responsible for the therapeutic effects of anti-Fas ligand antibody on viral myocarditis in mice. Zhonghua Er Ke Za Zhi 2005;43:920-4. [PubMed]
- Lin L, Zhang M, Yan R, et al. Inhibition of Drp1 attenuates mitochondrial damage and myocardial injury in Coxsackievirus B3 induced myocarditis. Biochem Biophys Res Commun 2017;484:550-6. [Crossref] [PubMed]
- Kischkel FC, Lawrence DA, Tinel A, et al. Death receptor recruitment of endogenous caspase-10 and apoptosis initiation in the absence of caspase-8. J Biol Chem 2001;276:46639-46. [Crossref] [PubMed]
- Soni H, Adebiyi A. TRPC6 channel activation promotes neonatal glomerular mesangial cell apoptosis via calcineurin/NFAT and FasL/Fas signaling pathways. Sci Rep 2016;6:29041. [Crossref] [PubMed]
- Fang J, Song XW, Tian J, et al. Overexpression of microRNA-378 attenuates ischemia-induced apoptosis by inhibiting caspase-3 expression in cardiac myocytes. Apoptosis 2012;17:410-23. [Crossref] [PubMed]
- Pirnia F, Schneider E, Betticher DC, et al. Mitomycin C induces apoptosis and caspase-8 and -9 processing through a caspase-3 and Fas-independent pathway. Cell Death Differ 2002;9:905-14. [Crossref] [PubMed]
- Prendergast GC. Mechanisms of apoptosis by c-Myc. Oncogene 1999;18:2967-87. [Crossref] [PubMed]
- O'Connell TD, Simpson RU. 1,25-Dihydroxyvitamin D3 regulation of myocardial growth and c-myc levels in the rat heart. Biochem Biophys Res Commun 1995;213:59-65. [Crossref] [PubMed]
- Nieminen AI, Partanen JI, Klefstrom J. c-Myc blazing a trail of death: coupling of the mitochondrial and death receptor apoptosis pathways by c-Myc. Cell Cycle 2007;6:2464-72. [Crossref] [PubMed]
- Guo Q, Hei Y, Chen Y. Zhonghua Shao Shang Za Zhi 2001;17:42-5. [The significance of the postburn expression of proto -- oncogenes c -- fos and c -- myc mRNA and proteins in rat myocardial cells]. [PubMed]
- Siwik DA, Chang DL, Colucci WS. Interleukin-1beta and tumor necrosis factor-alpha decrease collagen synthesis and increase matrix metalloproteinase activity in cardiac fibroblasts in vitro. Circ Res 2000;86:1259-65. [Crossref] [PubMed]
- Das S, Babick AP, Xu YJ, et al. TNF-alpha-mediated signal transduction pathway is a major determinant of apoptosis in dilated cardiomyopathy. J Cell Mol Med 2010;14:1988-97. [Crossref] [PubMed]


