Pulmonary vascular endothelial injury and acute pulmonary hypertension caused by COVID-19: the fundamental cause of refractory hypoxemia?
Introduction
COVID-19 was declared as a public health emergency of international concern in December of 2020 (1). Severe COVID-19 is characterized by refractory hypoxemia and multiple organ dysfunction syndrome (MODS). Until recently, we lacked a comprehensive understanding of the pathogenesis of COVID-19 and refractory hypoxemia. What we know has been limited in the virus infects human cells via the process of endocytosis by contacting ACE2 receptor (2), imbalance of the ACE2-RAAS-bradykinin axis (3), and the cytokine storm as it contributes to the progression of infection. In this case, considering the unique manifestations of hemoptysis, progressive pulmonary hypertension, and right heart failure in the mid-late stage, we envision that ACE2 receptor’s involvement in pulmonary vascular endothelium could be an important part of pathogenesis. Therefore, this finding may provide a new approach to improve patients’ clinical outcome. We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/cdt-20-429).
Case presentation
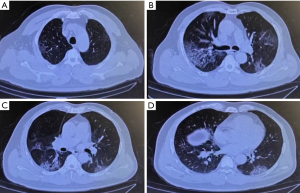
A 63-year-old man presented with a cough consisting of bloody phlegm on January 8, he reported traveling to Wuhan and Jingmen, Hubei province, China on December 29, 2019 and came back to Shenzhen on January 2, 2020. He developed a fever and shortness of breath within a few days, without any improvement after taking oseltamivir (7.5 mg bid). On January 13th, he was admitted to the emergency room because of hypoxemia. Chest CT showed mild bronchiectasis with pneumonia in progress. It was not difficult to determine that the chest CT lacking typical signs of pulmonary edema (Figure 1). He was immediately transferred to MICU as his breathing condition deteriorated. He denied medical history of COPD, heart disease, pulmonary hypertension. He had not experienced any chest tightness, edema or hemoptysis previously. He used to smoke 20 cigarettes a day for 20 years and quit smoking 20 years ago. He displayed shortness of breath, fever, and low pulse oxygen saturation but lacked rales in the lungs. Laboratory tests results showed a decline in the PaO2/FiO2 ratio to a minimal 59 mmHg, a slight increase in C-reactive protein level to 36.2 mg/L, and procalcitonin level to 0.18 ng/mL. Notably, the blood test results showed a decline of lymphocytes counts to 450/µL, while the total white cell number was 4,360/µL. In contrast, the cardiac troponin I level rose to 14.3 ng/mL.
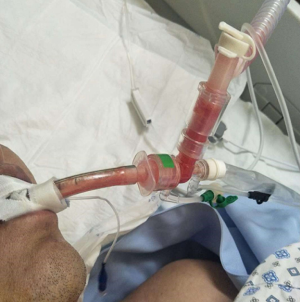
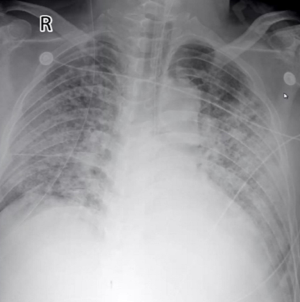
The patient was intubated after little improvement of HFNC and NIPPV therapy. The primary parameters of invasive ventilation: mode Duolevel, FiO2 100%, Phigh 26 cmH2O, Plow 14 cmH2O, PEEP 10 cmH2O, RR 18 bpm. Measured Pplat was 26 cmH2O in the VC mode. By conducting the bronchoscopy and bronchoalveolar lavage, we found massive thin bloody phlegm diffusely distributed in the airway, which was different from the pink frothy sputum, this indicated the existence of hemorrhagic alveolitis (Figure 2). The chest radiograph showed diffuse infiltration in bilateral parenchyma of the lungs with little pleural effusion (Figure 3). A few hours later, oliguria occurred, and the NT-proBNP level rose to 3,104 pg/mL. Subsequently, we implemented continuous renal replacement therapy (CRRT) and prone position. Following the PaO2/FiO2 ratio improved from 59 to 90 mmHg. His first sputum assays test to detect SARS-CoV-2 on RT-PCR was positive on January 15th. He was then transferred to Shenzhen Third People’s Hospital (national designated hospital for COVID-19).
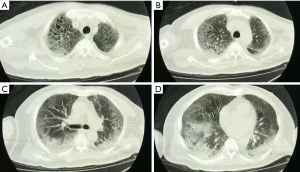
Using transthoracic echocardiography, pulmonary artery pressure (PAP) was noted to increase to 44 mmHg and left ventricular ejection fraction (LVEF) was noted to decreased to 32% with diffusely weak in ventricular wall movement (VWM), which suggested secondary fulminant myocarditis. After accepting methylprednisolone (80 mg per day), vitamin C (8 g per day) and supportive treatment, exudative lesions in both lungs was absorbed according to radiographs. His LVEF (54–68%) and VWM recovered five days later. PAP went down to 20 mmHg. Unfortunately, bloody phlegm and hypoxemia repeated and infiltration in the lungs developed into mixed lesions of lung parenchyma and interstitial (Figure 4). Dynamic monitoring by echocardiography showed constant tricuspid regurgitation and PAP rebounded to 36–50 mmHg, with the right ventricle (RV) expanding to the size of 74 mm × 55 mm (Table 1). No deep vein thrombosis was found on repeated ultrasounds of his extremities. After a period of VV-ECMO therapy (started on January 25th), the patient failed to fundamentally reverse the refractory hypoxia and died of septic shock and MODS on February 16, 2020.
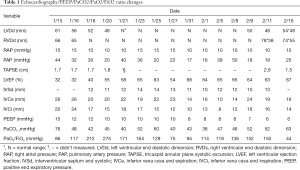
Full table
The manuscript was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient’s guardian for publication of this study and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Discussion
COVID-19 is an infectious disease caused by SARS-CoV-2, which infects human cells by binding to the ACE2 receptor (4). In most cases, focus is placed on the epithelial cells of the lungs, but this case aroused our attention because of the ACE2 receptor in the vascular endothelium.
What was the reason for acute pulmonary hypertension?
This case was the first confirmed COVID-19 case that presented severe ARDS when admitted to the hospital in our city (Luo H, Zhang S, Li YY, et al. Experience and advice from single medical center: How to rescue patients with serious COVID-19 and protect medical staffs without enough PPE. In the submission course to Cardiovasc Diagn Ther, 2020). Except for refractory hypoxemia, the remarkable clinical features were constant hemoptysis, worsening pulmonary hypertension, and acute right heart failure in the mid-late stage. First, the acute clinical manifestations mentioned above could not be explained merely by local chronic bronchiectasis. Second, fulminant myocarditis might cause pulmonary hypertension due to left heart disease, but could not explain the deterioration of pulmonary hypertension and the RV dysfunction after the left ventricular function recovered (Table 1). Third, ECMO improved hypoxemia and reduced the possibility of ARDS leading to continuous pulmonary vasospasm and acute pulmonary hypertension. Therefore, we focused on pulmonary fibrosis and pulmonary vascular disease. Meanwhile, the published pulmonary autopsy pathology of patients with COVID-19 did not indicate obvious inflammatory cells infiltrating the pulmonary interstitium but massive thrombosis existing in pulmonary vessels (5). This urges us to reconsider the role the pulmonary vascular system played in the process of pathogenesis and hypoxemia.
Did the right heart failure induced by high PEEP besides pulmonary hypertension?
Positive pressure ventilation will increase the RV afterload and lead to RV dysfunction when PEEP is >10 cmH2O in patients with PH or >15 cmH2O in patients without PH. But in this case, we set PEEP at about 10 cmH2O average or ≤10 cmH2O during ECMO (Table 1). There was no clear evidence showing processive right heart failure secondary to a reasonable PEEP setting.
What was the role of autoimmune responses in the case?
His chest CT presented ground-glass opacities in the subpleural area, indicating the lesions existed in the distal interstitial tissue and alveoli, which are similar to connective tissue disease associated interstitial lung disease (CTD-ILD). CTD-ILD usually manifested as chronic hemorrhagic capillary-alveolitis, pulmonary fibrosis, hypoxemia and pulmonary hypertension because of abnormal immunoreaction. Therefore, we speculated acute pulmonary vasculitis or capillaritis associated to immune damage and an inflammatory storm could exist in COVID-19. Being different from CTD-ILD, it progressed rapidly. Recently, a case report of PUMCH published in NEGM did reveal that there was a strong possibility of autoimmune involvement in the pathogenesis of COVID-19 (6).
What was the result of imbalance among the ACE2-RAAS-bradykinin axis?
Studies of SARS have revealed that ACE2, as a membrane-binding protein, is an important target for invasion of coronavirus. ACE2 receptor are distributed extensively among many organs. Especially in the lungs, ACE2 receptor expresses not only on type II alveolar epithelial cells and bronchiolar epithelial cells, but also on the smooth muscle cells of pulmonary vessels and vascular endothelium (7), which suggests that pulmonary vessels are also the targets of the virus attack. Once the coronavirus combines to the ACE2 receptor on pulmonary vessels, the ACE2-RAAS-bradykinin axis works improperly, inducing hyperbradykinemia, leading to high permeability of pulmonary vessels, hypotension, pulmonary fibrosis and pulmonary hypertension (8). Therefore, we reckon that SARS-CoV-2 infection can lead to hemoptysis and an acute fibrosis-like reaction in the early stages because of increasing leakage of red cell and plasma mucoprotein to the alveoli.
What message had been transmitted by autopsy?
We found that the pulmonary hypertension in this case was aggravated with pulmonary exudation, suggesting that pulmonary lesions were possibly the main cause of pulmonary hypertension. The first autopsy report of COVID-19 (5) in China showed normal-structured alveoli and massive thick excretion in the airway, implying the severe leakage secondary to destroy of pulmonary vessel structure rather than alveolar lesion. Therefore, the essence of refractory hypoxemia in COVID-19 patients might be a severe imbalance of the V/Q ratio due to vascular endothelial injury. Another study found blood clots in the placentas of 16 pregnant women who tested positive for SARS-CoV-2 (9). Combined with COVID-19 autopsy reports that found hyperemia, edema, and broadening of alveolar intervals, even hyaloid embolism in pulmonary vessels (10), we speculate that pulmonary hypertension probably correlates to pulmonary vasculitis, pulmonary capillaritis, thrombosis, and vessel remodeling.
Did the case show classic ARDS?
The respiratory mechanics of classic ARDS is often characterized by a significant increase in Pplat. The Pplat will be greater than 30 cmH2O in severe ARDS. However, the latest study in Italy found the Pplat of COVID-19 patients is not high enough (11). We came to a similar conclusion in our case with a Pplat of 26 cmH2O. Alveolar lesions in COVID-19 were not as serious as we expected, and thus, we suggested that the real serious lesions could be in pulmonary vessels. In other words, pulmonary vascular endothelium could be the main target of SARS-CoV-2 rather than alveolar epithelium in part of COVID-19 patients.
Summary
In this case, we have discussed the complication of hypoxemia and pulmonary hypertension induced by SARS-CoV-2. Most tend to draw conclusions of pulmonary vascular involvement because SARS-CoV-2 attacks not only alveolar epithelium but also pulmonary vascular endothelium. Hemoptysis might imply pulmonary vascular involvement in early stages due to ACE2 in the capillary endothelial affected by the virus. In later stages, thrombosis, pulmonary vascular remodeling, and pulmonary fibrosis due to a large amount of mucin exudation from pulmonary vessels into alveoli would aggravate pulmonary hypertension.
We think protective treatment of pulmonary vascular endothelium in COVID-19, just like low molecular weight heparin, fasudil, and reducing hyperbradykininemia deserve more attention. Plasma exchange may be effective to reduce the autoimmune response (12). Which becomes a hope to fundamentally alleviate refractory hypoxia. More biopsy and right cardiac catheterization are recommended to verify the speculations discussed above.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/cdt-20-429
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-429). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s guardian for publication of this study and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Coronavirus disease 2019 (COVID-19) Situation Report-68. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200328-sitrep-68-covid-19.pdf?sfvrsn=384bc74c_2
- Richardson P, Griffin I, Tucker C, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 2020;395:e30-1. [Crossref] [PubMed]
- Imai Y, Kuba K, Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005;436:112-6. [Crossref] [PubMed]
- Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature 2020.1-8.
- Liu L, Liu X, Wang RS. Report on gross observations of the cadaver system autopsy of a deceased COVID-19. Journal of Forensic Medicine 2020;36.
- Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N Engl J Med 2020;382:e38. [Crossref] [PubMed]
- Hamming I, Timens W, Bulthuis ML, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004;203:631-7. [Crossref] [PubMed]
- Zhang CJ, Yao Y. ACE2 is expected to be a new target for the treatment of pulmonary hypertension. W098318. 2014, Apr. Available online: http://www.365heart.com/show/98318.shtml?hipsvy=1rmcv1
- Shanes ED, Mithal LB, Azad HA, et al. Placental pathology in COVID-19. Am J Clin Pathol 2020;154:23-32. [Crossref] [PubMed]
- A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi 2020;49:E009.
- Gattinoni L, Coppola S, Cressoni M, et al. Covid-19 Does Not Lead to a "Typical" Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2020;201:1299-300. [Crossref] [PubMed]
- Lin L, Lu LF, Cao W, et al. Hypothesis for potential pathogenesis of SARS-CoV-2 infection–a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect 2020;9:727-32. [Crossref] [PubMed]