Diminished response to statins predicts the occurrence of heart failure after acute myocardial infarction
Introduction
Lowering low-density lipoprotein cholesterol (LDL-C) levels with a statin has become a mainstay of preventive therapeutic management in subjects with acute myocardial infarction (AMI). This is supported by randomized controlled trials (1,2) which have consistently demonstrated a significant reduction in atherosclerotic cardiovascular disease (ASCVD) in association with the degree of LDL-C control. In addition to this anti-atherosclerotic property, the potential effect of statins on heart failure (HF) events has been reported. The sub-analysis from the PROVE-IT TIMI22 study reported a lower occurrence of HF in subjects with acute coronary syndrome who achieved very low LDL-C levels under intensive statin therapy (3). One recent meta-analysis also showed that statins modestly reduced the occurrence of HF, whereas other randomized controlled trials failed to prove the reduction of HF events under statin therapy (4). These inconsistent observations suggest that the extent of HF risk reduction after statin therapy may be heterogenous due to other factors.
The degree of LDL-C reduction with statins varies across individuals. One secondary data analysis of the JUPITER trial reported that 53.6% of healthy subjects did not exhibit an expected response to 20 mg rosuvastatin (5). In another study, poor reduction in LDL-C levels (=% change in LDL-C <15%) was observed in 20% of subjects with coronary artery disease receiving statins (6). Given that an elevated LDL-C level promotes oxidative stress and endothelial dysfunction associated with HF, we hypothesized that the degree of response to statin therapy may affect HF risks after statin therapy. Therefore, the current study sought to investigate the frequency of HF events in patients with AMI according to their response to statin. We present the following study in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/cdt-20-415).
Methods
Study sample
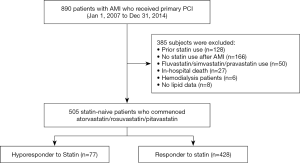
We retrospectively analyzed a total of 890 consecutive patients with de novo AMI who received primary percutaneous coronary intervention (PCI) from January 2007 to December 2014 at the National Cerebral and Cardiovascular Center in Suita, Japan. The diagnosis of myocardial infarction was based on the European Society of Cardiology/American College of Cardiology Foundation/American Heart Association/World Heart Federation Task Force for the Universal Definition of Myocardial Infarction (7). Of these, the following patients were excluded: patients who received a statin prior to AMI (n=128), those who did not receive statin therapy after AMI (n=166), subjects receiving a statin except atorvastatin/rosuvastatin/pitavastatin (n=50), those with in-hospital death after AMI (n=27), subjects with hemodialysis due to end-stage renal disease (n=6), and patients who did not have lipid profile data (n=8). The remaining 505 statin-naïve subjects were included into the current analysis (Figure 1). The research protocol was approved by the ethics committee of our institution (M24-055-6). The research was conducted in accordance with the Declaration of Helsinki. Informed consent was not obtained in each subject due to the observational analysis of hospitalized patients. However, the current study was posted on the website of our institution (http://www.ncvc.go.jp/hospital/pub/clinical-research/untersuchung/untersuchung-78.html) to inform its detail and ensure that patients could refuse inclusion into the current analysis. When we contacted with participants by a mail or telephone, we explained the study subjects and then obtained informed consent.
Definition of hyporesponse to statin
In the current study, atorvastatin, rosuvastatin or pitavastatin was commenced within 24 hours after the completion of primary PCI. The selection of statin and its dose was left to each physician’s discretion. Physicians managed lipid-lowering therapy according to the Japan Atherosclerosis Society guideline for the secondary prevention of ASCVD. In brief, achieving LDL-C <100 [2007–2017] and 70 [2017–currently] mg/dl is recommended as a therapeutic goal in Japanese patients. If this goal is not achievable, escalation of the dose of a statin and/or commencement of other lipid-lowering therapy is considered (8,9). LDL-C was measured on admission and at 1 month after the completion of primary PCI in all subjects. Hyporesponse to statin was defined as a percentage of reduction in LDL-C <15% from baseline to 1 month after statin treatment (6). High-intensity statin was defined as atorvastatin ≥20 mg, rosuvastatin ≥10 mg and pitavastatin ≥4 mg (10).
PCI procedure
After identification of the culprit lesion on diagnostic coronary angiography, primary PCI was performed with the use of 6- or 7-French catheters, followed by the commencement of dual antiplatelet therapy, as previously reported (11). All procedural decisions, including device selection, the use of mechanical support, and adjunctive pharmacotherapy were made according to the discretion of the individual PCI operator.
Outcomes
The primary outcome was the occurrence of hospitalization for HF following statin therapy. The diagnosis of HF was made according to the Framingham criteria (12). The secondary outcome was a composite of cardiovascular death and hospitalization for HF after statin use. A composite of cardiovascular death, non-fatal myocardial infarction, stroke and unstable angina pectoris requiring revascularization was also analyzed. These outcomes were obtained through reviewing the medical records of each hospital visit, and, when necessary, through a questionnaire by mail and telephonic follow-up.
Statistical analysis
Descriptive statistics were summarized according to the presence or absence of hyporesponse to statin therapy. Results are presented as percentages for categorical variables and mean ± standard deviation for continuous variables. When variables were not normally distributed, their results were described as median (interquartile range). Clinical characteristics were compared using Student’s t-test or Wilcoxon rank-sum test for continuous variables as appropriate. For categorical variables, the Pearson chi-square test or Fisher’s exact test was used. Changes in LDL-C levels were compared by analysis of covariance, after controlling for baseline values. Wilcoxon signed rank test was used to compare changes in the CRP level between two groups.
The Kaplan-Meier method was used to estimate survival curves for primary and secondary outcomes and the log-rank test was used to assess differences between responders and hyporesponders to statins. Unadjusted hazard ratios for HF were calculated by a univariate Cox proportional hazards model. Adjusted hazard ratios were calculated by a multivariate Cox proportional hazards model with a P value of 0.10. This model included the following variables: age, gender, body mass index, type 2 diabetes mellitus, Killip class, left ventricular ejection fraction, β-blocker use, high-intensity statin use, and statin hyporesponse. A multivariate Cox proportional hazards model using stepwise selection with a P value of 0.10 was also used. This model included age, Killip class, left ventricular ejection fraction and statin hyporesponse.
To further account for significant differences in baseline clinical characteristics between statin responders and hyporesponders, a propensity score-matched analysis was performed. The propensity score was estimated using logistic regression models, with statin responders or hyporesponders as the outcome and baseline clinical demographics as predictors (covariates: age, gender, body mass index, hypertension, type 2 diabetes mellitus, Killip class, left ventricular ejection fraction, baseline LDL-C and use of high-intensity statin and β-blockers). Statin responders and hyporesponders were matched by propensity score on a 2:1 basis by using the nearest-neighbour matching method the R package “Matching” using the R statistical software. In addition, inverse probability of treatment weights (IPTW) based on the calculated propensity score as mentioned above was applied to evaluate the association between statin hyporesponse and HF by estimating the average treatment effects (ATEs) (13). All P values <0.05 were considered statistically significant. All analyses were performed with JMP version 13.0.0 (SAS Institute, Cary, NC, USA), STATA version 15 (StataCorp, College Station, TX, USA) and the R statistical software.
Results
Clinical characteristics
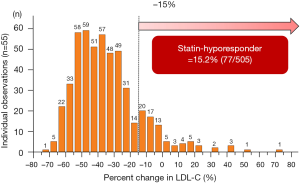
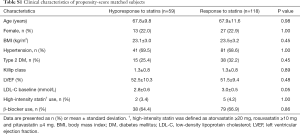
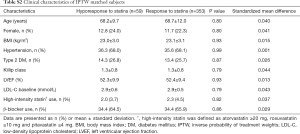
In the current study, 15.2% (77/505) of study subjects showed hyporesponse to statins (Figure 2). Table 1 summarizes baseline clinical demographics. Statin hyporesponders were less likely to have a history of dyslipidemia (57.1% vs. 70.8%, P=0.02) and type 2 diabetes mellitus (22.1% vs. 36.0%, P=0.02). Body mass index (BMI) was lower in statin hyporesponder (23.5±3.4 vs. 24.5±3.6 kg/m2, P=0.04). Clinical presentation was ST-segment elevation myocardial infarction in over 85% of each patient group (P=0.86). There were no significant differences in the proportion of Killip class ≥ II (P=0.43), the number of patients who achieved final Thrombolysis In Myocardial Infarction III flow (P=0.84), left ventricular ejection fraction (P=0.08) and the level of peak creatine phosphokinase between groups (P=0.15).
Full table
Regarding the use of statins and other established medical therapies (Table 1), hyporesponders to statin were more likely to receive atorvastatin (48.1% vs. 28.0%, P=0.0008), and less likely to be treated with rosuvastatin (16.9% vs. 40.4%, P<0.0001), whereas the use of pitavastatin was similar between two groups (P=0.60). The proportion of patients receiving ≥20 mg atorvastatin (0.0% vs. 0.7%, P=1.00), ≥10 mg rosuvastatin (1.3% vs. 4.9%, P=0.23) and ≥4 mg pitavastatin (1.3% vs. 4.0%, P=0.33) was comparable between hyporesponders and responders. While β-blocker use was less frequent in statin hyporesponders (61.0% vs. 75.9%, P=0.01), the use of other established medical therapies did not differ in two groups (Table 1).
Changes in LDL-C and CRP
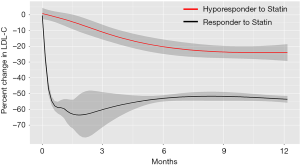
In the current study, 98% of entire study subjects continued to receive prescription of a statin, and there was no significant difference between two groups (hyporesponder: 100% vs. responder: 96.9%, P=1.00). In addition, the frequency of further intensification of statin therapy (statin dose escalation and/or greater intensity statin use) was not significantly different between hyporesponders and responders (21.4% vs. 24.2%, P=1.00). Table 2 shows LDL-C and CRP levels on admission and at 1 month after the commencement of statin therapy. A lower LDL-C level at baseline was observed in hyporesponders to statin therapy (2.9±0.7 vs. 3.6±0.8 mmol/L, P<0.001). Predictably, these patients showed a higher LDL-C level at 1 month (2.9±0.6 vs. 2.1±0.5 mmol/L, P<0.001) accompanied by its smaller reduction compared to responders (+2.1%±17.7% vs. −41.2%±12.3%, P<0.001). Serial change in percent reduction of LDL-C beyond 1 month was illustrated by Figure 3. In statin hyporesponders, a smaller percent change in LDL-C continued to exist at 6, 12 and 48 months (Figure 3). In addition, percent change in LDL-C was still significantly different in two groups at 6 (P<0.0001) and 12 months (P<0.0001). This comparison showed borderline significance at 48 months (P=0.05).

Full table
With regard to CRP levels, baseline and 1-month CRP levels did not differ between the groups (Table 2).
Occurrence of heart failure
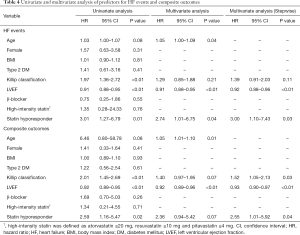
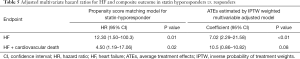
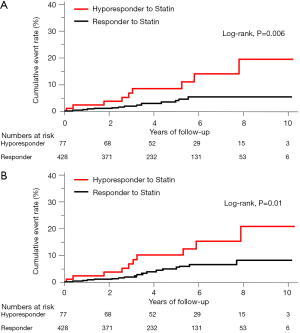
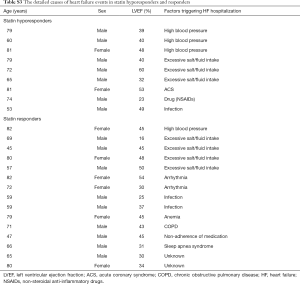
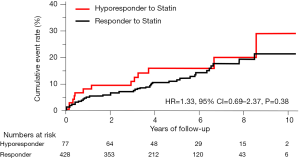
The incidence of HF events during the observational period [median =4.4 years, interquartile range (IQR): 2.9 to 6.8 years] in responders and hyporesponders to statin is summarized in Tables 3,4,5 and Figure 4. The frequency of β-blocker use at its maximal dose was similar in two groups (20.8% vs. 17.5%, P=0.68). HF and cardiac-related death occurred in 24 and 8 subjects, respectively (Table 3). Hyporesponse to statin was associated with a 3.01- [95% confidence interval (CI): 1.27–6.79, P=0.01] and 2.59-fold (95% CI: 1.16–5.47, P=0.02) greater likelihood of experiencing HF and a composite of HF and cardiovascular death (Table 4, Figure 4A,B), respectively. On multivariate Cox proportional hazard model analysis as well as the stepwise regression analysis, hyporesponse to statin continued to predict the occurrence of HF and a composite outcome (Table 4). The propensity score-matching analysis was conducted to further evaluate the predictive ability of hyporesponse to statin for HF events. Following the selection of 59 hyporesponders and 118 responders with matched baseline characteristics (Table S1), a greater risk for HF and composite outcome was observed in statin hyporesponders (HF: HR =12.30, 95% CI: 1.50–100.3, P=0.01, HF and cardiovascular death: HR =4,50, 95% CI: 1.19–17.06, P=0.02). Even in the IPTW analysis with ATEs, the association between statin hyporesponders and an increased risk of HF remained (coefficient =7.02, 95% CI: 2.29–21.58, P=0.0006, Table 5 and Table S2). Additionally, statin hyporesponse tended to be associated with a higher frequency of composite outcome (coefficient =3.05, 95% CI: 0.86–10.82, P=0.08, Table 5). The detailed cause of heart failure events in statin hyporesponders and responders is summarized by Table S3. With regard to the occurrence of atherosclerotic cardiovascular events, there was no significant difference between two groups (HR =1.33, 95% CI: 0.69–2.37, P=0.38, Figure S1).

Full table
Full table
Full table
Full table
Full table
Full table
Based on hazard ratio of heart failure as 7.02 obtained by IPTW analysis, the power of the analysis was calculated as 87.7% with an alpha level of 5% and 505 patients. With regard to the sample size calculation, 332 subjects were required, with an alpha level of 5% and hazard ratio of heart failure as 7.02, which gave power of 80%.
Heart failure risk and the degree of response to a statin
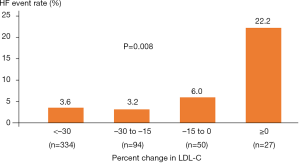
The occurrence of HF was compared in subjects stratified according to the percent reduction in LDL-C levels following statin therapy (Figure 5). An increased frequency of HF event was observed in association with the extent of percent reduction in LDL-C levels (P=0.008). In particular, the risk of HF events increased in AMI subjects who did not obtain any LDL-C reduction despite statin therapy (Figure 5).
Discussion
The current findings provide important insights into the association between the response to a statin and the occurrence of HF events. Despite statin therapy, about 15% of AMI subjects did not show an adequate lowering of LDL-C levels. Furthermore, this poor response to a statin increased the risk of HF following AMI. Our findings indicate that hyporesponse to a statin may be a potential factor associated with HF events in patients with AMI.
Statin hyporesponse causes high LDL-C levels, which is an important driver to propagate proatherogenic damage within vessel walls. Mechanistically, circulating LDL has been reported to cause eNOS uncoupling, which stimulates superoxide production and promotes endothelial dysfunction (14,15). In addition, one recent study found that LDL-C level was independently associated with oxidative markers and endothelial function (16). Given that endothelial dysfunction increases microvascular resistance and extravascular fluid accumulation (17), these LDL-C-derived effects in statin hyporesponders may elevate a risk of HF (18,19). As shown in Figure 5, the frequency of HF varies according to the degree of LDL-C reduction under statin therapy. These observations suggest that suboptimal LDL-C lowering due to hyporesponse to a statin may negatively affect vascular tone and fluid retention, which potentially influence the ability of statins to modify HF risk following AMI.
While statins have been shown to possess anti-inflammatory properties (20,21), whether this effect is diminished in statin hyporesponders remains to be determined. In our analysis, an increase in CRP levels (25.0%) was observed in statin hyporesponders, although this difference did not meet statistical significance (Table 2). Another study identified a trend towards a smaller percentage of reduction in CRP levels in statin hyporesponders (6). These findings suggest that the property of statins to modulate inflammatory activity may be also diminished. Since a systemic inflammatory state has been considered to cause HF via increased endothelial adhesion molecules expression and reactive oxygen species production (22,23), the unfavourable control of inflammatory activity may be another driver for the occurrence of HF events in statin hyporesponders.
The precise mechanism of impaired LDL-C reduction in statin hyporesponders remains unknown. One of speculative mechanisms is the statin-mediated increase in circulating proprotein convertase subtilisin/kexin type 9 (PCSK9) level. PCSK9 is a serine protease degrading LDL receptor, which induces an increase in circulating LDL-C (24). In addition to its property associated with LDL metabolism, PCSK9 has been shown to promote inflammation and worsen endothelial permeability, including nitric oxide production (25,26). Of note, the circulating PCSK9 level itself has been shown to be a significant predictor of a composite endpoint of all-cause death and HF hospitalization by the BIOSTAT-CHF subanalysis (27). Although the current study did not measure PCSK9 levels, a potential elevation of PCSK9 levels in statin hyporesponders may be an important substrate causing its diminished effect.
We observed that 15.2% of AMI subjects did not favorably respond to statin therapy. Given that lowering LDL-C is a mainstay of preventive management in the setting of AMI, considering additional lipid-lowering therapy is required in AMI subjects with poor response to a statin. Recent clinical trials showed clinical efficacy of ezetimibe, evolocumab and alirocumab on lipid parameters as well as cardiovascular outcomes. As such, evaluation of response to a statin could provide an opportunity to select appropriate agent for achieving optimal lipid control after AMI in each individual.
The present study has several limitations. Firstly, this was a retrospective observational study at a single center that included a relatively small number of statin hyporesponders and cardiac events. Secondly, the type and dose of statins and cardioprotective drugs were decided by each physician. Thirdly, we analyzed AMI subjects who were hospitalized from 2007 to 2014. In this study period, the guideline-recommended LDL-C goal in Japan was LDL-C <100 mg/dL, and the use of statin was not clearly recommended. This may affect a lower frequency of high-intensity statin use. Fourthly, the current study did not collect data about compliance of a statin because it is a retrospective analysis. Fifthly, serum PCSK9 and other biomarkers associated with inflammation and endothelial function have not been evaluated in this study. Lastly, β-blocker was less frequently used in statin hyporesponders, which may affect their HF outcomes. However, even after adjusting for baseline medication use, the relationship between statin hyporesponse and HF events remained.
In conclusion, the present study revealed that 15.2% of patients with AMI showed a poor response to statins. This reduced efficacy of statins caused a suboptimal control of LDL-C after AMI. Furthermore, hyporesponse to statins was associated with an increased risk of HF. Our findings highlight statin hyporesponsiveness as a phenotype with an increased risk of HF which requires additional preventive therapy.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed STROBE reporting checklist. Available at http://dx.doi.org/10.21037/cdt-20-415
Data Sharing Statement: Available at http://dx.doi.org/10.21037/cdt-20-415
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-415). YK serves as an unpaid editorial board member of Cardiovascular Diagnosis and Therapy from Jul 2019 to Jun 2021. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The research protocol was approved by the ethics committee of our institution (M24-055-6). The research was conducted in accordance with the Declaration of Helsinki. Informed consent was not obtained in each subject due to the observational analysis of hospitalized patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996;335:1001-9. [Crossref] [PubMed]
- Cannon CP, Braunwald E, McCabe CH, et al. Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495-504. [Crossref] [PubMed]
- Scirica BM, Morrow DA, Cannon CP, et al. PROVE IT-TIMI 22 Investigators. Intensive statin therapy and the risk of hospitalization for heart failure after an acute coronary syndrome in the PROVE IT-TIMI 22 Study. J Am Coll Cardiol 2006;47:2326-31. [Crossref] [PubMed]
- Preiss D, Campbell RT, Murray HM, et al. The effect of statin therapy on heart failure events: a collaborative meta-analysis of unpublished data from major randomized trials. Eur Heart J 2016;36:1536-46.
- Ridker PM, Mora S, Rose L, et al. JUPITER Trial Study Group. Percent reduction in LDL cholesterol following high-intensity statin therapy: potential implications for guidelines and for the prescription of emerging lipid-lowering agents. Eur Heart J 2016;37:1373-9. [Crossref] [PubMed]
- Kataoka Y, St. John J, Wolski K, et al. Atheroma progression in hyporesponders to statin therapy. Arterioscler Thromb Vasc Biol 2015;35:990-5. [Crossref] [PubMed]
- Thygesen K, Alpert JS, Jaffe AS, et al. Joint ESC/ACCF/AHA/WHF Task Force for Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Eur Heart J 2012;33:2551-67. [Crossref] [PubMed]
- Teramoto T, Sasaki J, Ueshima H, et al. Executive summary of Japan Atherosclerosis Society (JAS) guideline for diagnosis and prevention of atherosclerotic cardiovascular diseases for Japanese. J Atheroscler Thromb 2007;14:45-50. [Crossref] [PubMed]
- Teramoto T, Sasaki J, Ishibashi S, et al. Japan Atherosclerosis Society (JAS). Comprehensive risk management for the prevention of cardiovascular disease: executive summary of the Japan Atherosclerosis Society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan – 2012. J Atheroscler Thromb 2013;20:603-15. [Crossref] [PubMed]
- Harada-Shiba M, Ako J, Arai H, et al. Prevalence of familial hypercholesterolemia in patients with acute coronary syndrome in Japan: Results of the EXPLORE-J study. Atherosclerosis 2018;277:362-8. [Crossref] [PubMed]
- Arakawa K, Yasuda S, Hao H, et al. Significant association between neutrophil aggregation in aspirated thrombus and myocardial damage in patients with ST-segment elevation acute myocardial infarction. Circ J 2009;73:139-44. [Crossref] [PubMed]
- McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971;285:1441-6. [Crossref] [PubMed]
- Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015;34:3661-79. [Crossref] [PubMed]
- Shaul PW. Regulation of endothelial nitric oxide synthase: location, location, location. Annu Rev Physiol 2002;64:749-74. [Crossref] [PubMed]
- Vergnani L, Hatrik S, Ricci F, et al. Effect of native and oxidized low-density lipoprotein on endothelial nitric oxide and superoxide production: key role of L-arginine availability. Circulation 2000;101:1261-6. [Crossref] [PubMed]
- Al-Benna S, Hamilton CA, McClure JD, et al. Low-density lipoprotein cholesterol determines oxidative stress and endothelial dysfunction in saphenous veins from patients with coronary artery disease. Arterioscler Thromb Vasc Biol 2006;26:218-23. [Crossref] [PubMed]
- Münzel T, Daiber A, Ullrich V, et al. Vascular consequences of endothelial nitric oxide synthase uncoupling for the activity and expression of the soluble guanylyl cyclase and the cGMP-dependent protein kinase. Arterioscler Thromb Vasc Biol 2005;25:1551-7. [Crossref] [PubMed]
- Ferrari R, Bachetti T, Agnoletti L, et al. Endothelial function and dysfunction in heart failure. Eur Heart J 1998;19 SupplG:G41-7.
- Marti CN, Gheorghiade M, Kalogeropoulos AP, et al. Endothelial dysfunction, arterial stiffness, and heart failure. J Am Coll Cardiol 2012;60:1455-69. [Crossref] [PubMed]
- Ray KK, Cannon CP. The potential relevance of the multiple lipid-independent (pleiotropic) effects of statins in the management of acute coronary syndromes. J Am Coll Cardiol 2005;46:1425-33. [Crossref] [PubMed]
- Oesterle A, Laufs U, Liao JK. Pleiotropic Effects of Statins on the Cardiovascular System. Circ Res 2017;120:229-43. [Crossref] [PubMed]
- Westermann D, Lindner D, Kasner M, et al. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail. 2011;4:44-52. [Crossref] [PubMed]
- Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 2000;86:494-501. [Crossref] [PubMed]
- Bergeron N, Phan BA, Ding Y, et al. Proprotein convertase subtilisin/kexin type 9 inhibition: a new therapeutic mechanism for reducing cardiovascular disease risk. Circulation 2015;132:1648-66. [Crossref] [PubMed]
- Ding Z, Liu S, Wang X, et al. Hemodynamic shear stress via ROS modulates PCSK9 expression in human vascular endothelial and smooth muscle cells and along the mouse aorta. Antioxid Redox Signal 2015;22:760-71. [Crossref] [PubMed]
- Tang Z, Jiang L, Peng J, et al. PCSK9 siRNA suppresses the inflammatory response induced by oxLDL through inhibition of NF-κB activation in THP-1-derived macrophages. Int J Mol Med 2012;30:931-8. [Crossref] [PubMed]
- Bayes-Genis A, Núñez J, Zannad F, et al. The PCSK9-LDL Receptor Axis and Outcomes in Heart Failure: BIOSTAT-CHF Subanalysis. J Am Coll Cardiol 2017;70:2128-36. [Crossref] [PubMed]