Incidence of myocardial injury in coronavirus disease 2019 (COVID-19): a pooled analysis of 7,679 patients from 53 studies
Introduction
Since December 2019, coronavirus disease 2019 (COVID-19) caused by the newly discovered severe acute respiratory syndrome coronavirus 2 (SAR-CoV-2) has been recognized as a major public health issue due to rapidly global pandemic, resulting in 7,823,289 confirmed infections and 431,541 deaths worldwide by 15 June 2020 (1). Previous studies have described the main findings of clinical and epidemiological characteristics in COVID-19 patients (2-4). With the increase of confirmed cases and the accumulation of clinical data, the cardiovascular manifestations caused by COVID-19 has raised concern. Myocardial injury, defined as elevated levels of troponin or creatine kinase isoenzyme (CK-MB) regardless of new abnormalities in electrocardiography and echocardiography, have been reported with the rate of 7.2% in the initial COVID-19 study (4). Two recent studies presented 19.7% to 27.8% of patients with COVID-19 had acute myocardial injury (5,6). Obviously, incidence of myocardial injury in this viral infection remains uncertain. The pathophysiology of COVID-associated myocardial injury have not well established but likely involve the direct damage to cardiomyocytes, systemic inflammation, myocardial interstitial fibrosis, interferon mediated immune response, exaggerated cytokine response, in addition to coronary plaque destabilization, and hypoxia (7). Apart from COVID-19 itself, there are other factors associated with myocardial injury in these patients, which include cardiovascular risk factors (smoking, hypertension, obesity, physical inactivity, advanced age), severe forms of the disease and medications such as hydroxychloroquine or chloroquine (7-9). Currently published meta-analyses have reported that more myocardial injury happened in severe COVID-19 patients, which was subsequently associated with deteriorative outcomes [mortality and need for intensive care unit (ICU) care] (10-12). Nevertheless, no study until now have given a full picture for myocardial injury incidence in patients with COVID-19. The present study therefore summarized all available evidence for a comprehensive and rigorous systematic review focused on myocardial injury incidence in COVID-19. In addition, to state the case-fatality rate related to cardiac injury, variations of myocardial injury incidence were also examined by disease severity (non-survivors, severe patients, and non-severe patients). We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/cdt-20-535).
Methods
This systematic review and meta-analysis was established according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement. The authors declare that all supporting data are available within the article and in the Supplementary file.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The ethical approval and consent are not required because no patient-level data is involved for this systematic review and meta-analysis.
Data sources and searches
Relevant studies were identified by performing English-language searches of PubMed, Embase, Cochrane Library databases (through April 24, 2020) as well as Chinese-language searches of China National Knowledge Infrastructure (CNKI), China Science and Technology Journal Database (VIP), WANFANG databases (through April 23, 2020) using the search terms related to COVID-19. The full search strategy is outlined in Table S1. Preprint articles were retrieved from the websites of MedRxiv (https://www.medrxiv.org), BioRxiv (https://www.biorxiv.org), and SSRN (https://www.ssrn.com) (through April 24, 2020). Manual search was also conducted by screening the reference lists of inclusive studies and relevant meta-analysis.

Full table
Study selection and outcomes
Studies of any types [case series study, cross-sectional study, case control study, cohort study, or randomized controlled trial (RCT)] were eligible for inclusion if they included SARS-CoV-2 infected adult patients; reported the qualitative data of cardiac specific biomarkers (troponin or CKMB); or reported the data of myocardial injury with detailed definition. Studies were excluded if they did not report defined myocardial injury indexes or published in meta-analysis or case report. Because of the difficulty to estimate the potentially repetitive patients, all the studies met the inclusion criteria were available for meta-analysis. Two authors (ZG and CZ) independently reviewed each title and abstract, and assessed full texts of retrieved studies, with any disagreements being resolved via consultation with a third author (JP). The primary outcomes of this study were the incidence of myocardial injury in COVID-19 and corresponding relative risk (RR) in comparison between severe and non-severe patients. COVID-19 patients was the laboratory diagnosis using real time reverse transcription-polymerase chain reaction (RT-PCR) assay or clinical diagnosis based on the Guidance for COVID-19 (7th edition) released by the National Health Commission of China. Myocardial injury was defined as serum levels of troponin or CK-MB above the 99th percentile upper reference limit, regardless of new abnormalities in electrocardiography and echocardiography. Severe patients were judged according to the Guidance for COVID-19 (7th edition) released by the National Health Commission of China (13).
Data extraction and quality assessment
Two authors (ZG and CZ) independently extracted the data using a priori designed form: which included study characteristics (study name, study source, regions, detailed hospital), patient characteristics (included period, illness severity, diagnosis standard for COVID-19, myocardial injury definition and its cut-off value), clinical characteristics (age, gender, smoking, and the comorbidities of hypertension, diabetes, cardiovascular disease (CVD), cerebrovascular disease, chronic pulmonary disease, chronic kidney disease, liver disease, and cancer), and data on cardiac injury (occurrence number and total number). The methodological quality of included RCTs was evaluated according to Cochrane Collaboration Risk of Bias Tool (14). The methodological quality of each included observational studies was assessed according to the Newcastle-Ottawa Scale (NOS) (15). To fit in our study design, the NOS was modified with totally 8 scores and the following 6 dimensions: representative of the sample; ascertainment of the exposure; ascertainment of the outcome; ascertainment of the outcome for quality control; control for factors of age and gender; and control for factors related to myocardial injury. A study can be awarded a maximum of 1 point for the first 4 dimensions and a maximum of 2 points for the last 2 dimensions (control for factors of age and gender: 1 point for age and 1 point for sex; control for factors related to myocardial injury: 1 point for reporting 1 or 2 comorbidities and 2 points for reporting ≥3 comorbidities). The total scores of ≥5 points represented a relatively good quality.
Data synthesis and statistical analysis
A random-effects (DerSimonian and Laird method) meta-analysis was used to calculate the pooled incidence of myocardial injury with 95% confidence intervals (95% CIs). Likewise, RRs of myocardial injury comparing severe with non-severe patients was performed. Heterogeneity among studies was assessed using the Cochran Q test and I2 index, with I2 >50% representing considerable heterogeneity (16). Subgroup analysis was conducted by the severity of illness (non-survivors, severe patients, and non-severe patients). The interaction analysis (P for interaction) using Cochran’s Q test were applied to evaluate the risk difference of different illness severity (17). Interaction is referred to as effect modification, which investigates whether the effect of intervention in the primacy outcome varied between the subgroup such as disease severity. A leave-1-out sensitivity analysis for each meta-analysis was applied to explore whether a single study had an excessive influence on myocardial injury incidence. To strengthen the robustness of the results, further serial sensitivity analyses were conducted by including studies that real time RT-PCR assay was performed using a SARS-CoV-2 nucleic acid detection, or studies that used troponin or electrocardiography or echocardiography as definition of myocardial injury, or excluding studies that involved potentially repetitive patients in the same hospital with period within range of other studies; or excluding studies that sample size were <50. To address the potential risk factors associated with myocardial injury, all preexisting cardiovascular risk factors or established diseases will be taken into consideration in the meta-regression. As a rule, at least 25% data points should be available for each variable in univariable meta-regression. The presence of publication bias was evaluated qualitatively by funnel plots and quantitatively by Begg’s test and Egger’s test when more than 10 studies were available in a single analysis (18). Trim and fill method was used to deal with the publication bias. The trim and fill method requires no assumptions about the mechanism that lead to publication bias, provides an estimate of the number of missing studies, and also provides an estimated intervention effect to adjust the publication bias. Data were analyzed using Stata version 13.0 (StataCorp., College Station, TX, USA).
Results
Study selection and study characteristics
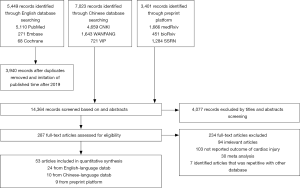
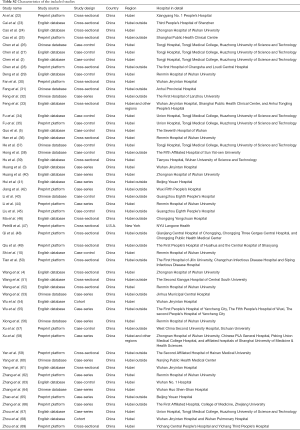
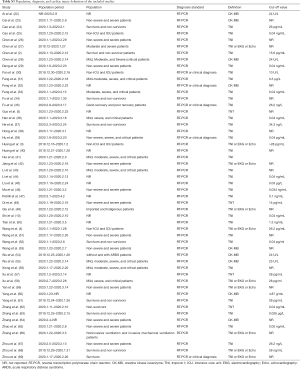
As outlined in Figure 1, initial search identified 5,449 records from English-language databases, 7,023 from Chinese-language databases, and 3,401 from preprint platform; 1,509 duplicates were removed and 14,077 records were excluded by screening titles and abstracts; the remaining 287 full-text articles were reviewed and 234 articles were excluded with the following reasons: studies were irrelevant (n=94), studies did not report outcome of myocardial injury (n=103), studies were meta-analyses, and studies was repetitive with other database (n=7). Finally, 53 studies involving 7,679 COVID-19 patients were included, with 24 from English-language databases, 10 from Chinese-language databases, and 19 from preprint platform. Among them, 21 studies (39%) were cross-sectional studies, 16 (30%) were case-series studies, 14 (26%) were case-control studies, and 2 (5%) were cohort studies. Twenty-eight studies (53%) were conducted in Hubei, 22 (41%) in regions outside Hubei, 2 (4%) in both Hubei and other regions, and 1 in New York (Table S2). The majority of studies (48/53, 91%) used RT-PCR method for confirming COVID-19. The remaining 5 studies used RT-PCR method or clinical diagnosis definition for confirming COVID-19. Thirty-three studies (62%) used troponin, 11 (21%) applied troponin or electrocardiography or echocardiography, and the remaining 9 (17%) employed CK-MB as cardiac injury definition (Table S3). The number of included COVID-19 patients varied from 8 to 1,327. The mean age was 54 years and the percentage of male was 54.1%. Other detailed information on comorbidities is summarized in Table S4.
Full table
Full table
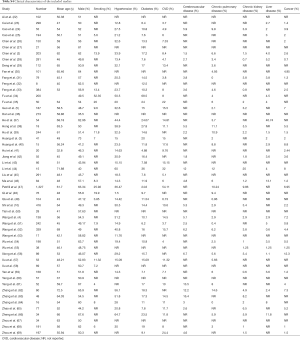
Full table
Study quality
All included studies satisfied the following risk bias items: representative of the sample; ascertainment of the exposure; ascertainment of the outcome; and control for factors of age and gender. Twenty-three studies (43%) defined the myocardial injury in the text (ascertainment of the outcome for quality control); 37 studies (70%) reported more than 3 comorbidities (2 points) and 11 studies (21%) reported 1 or 2 comorbidities (1 point). Eventually, all 53 studies were rated as relatively good quality (Table S5).
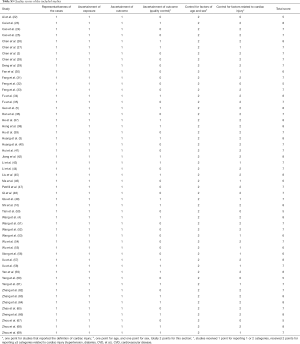
Full table
Incidence of myocardial injury
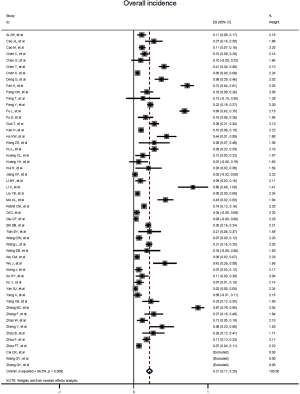
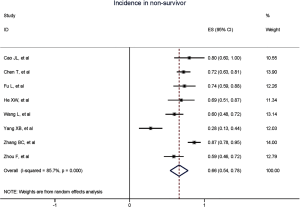
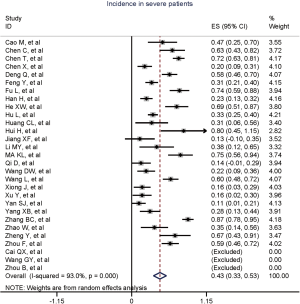
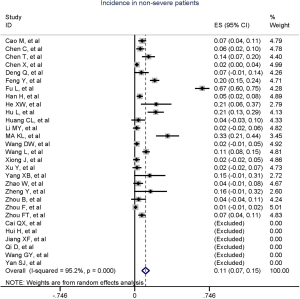
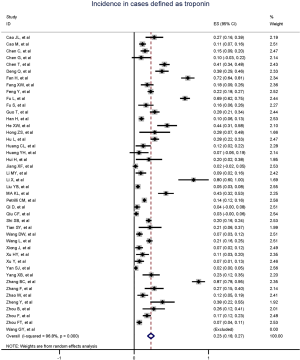
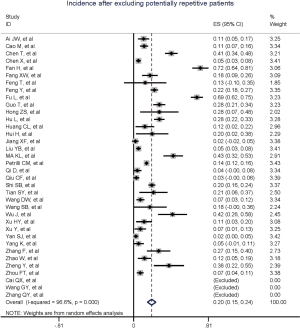
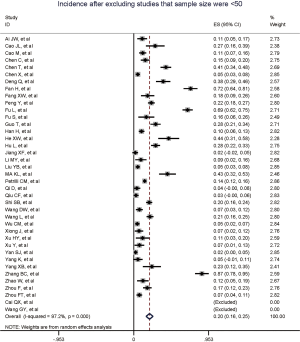
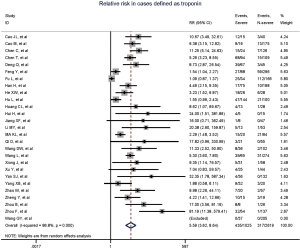
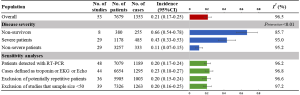
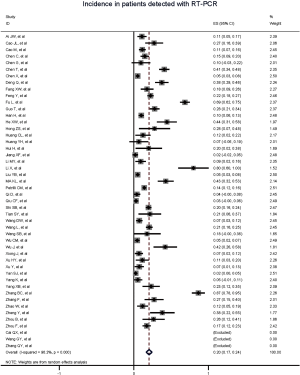
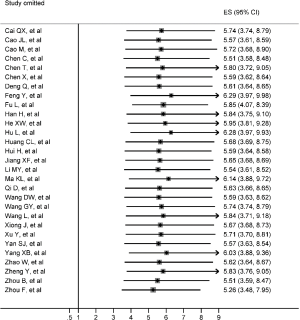
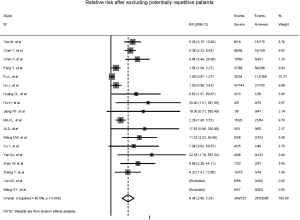
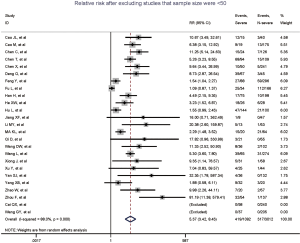
Figure 2 gives the full picture of myocardial injury incidence. The overall pooled incidence of myocardial injury was 21% (95% CI, 17–25%; I2, 96.5%; Figure S1). For severity of illness, the highest incidence of myocardial injury was found in non-survivors (66%; 95% CI, 54–78%; I2, 85.7%; Figure S2), followed by severe patients (43%; 95% CI, 33–53%; I2, 93.0%; Figure S3) and non-severe patients (11%; 95% CI, 7–15%; I2, 95.2%; Figure S4), with significant difference (Pinteraction <0.01). Sensitivity analyses by removing a single study at 1 time; or including studies that patients were detected with RT-PCR assay; or including studies that cases were defined as troponin; or excluding studies that involved potentially repetitive patients or sample size were <50 confirmed the robustness of primacy results (Table S6 and Figures S5,S6,S7,S8).
Full table
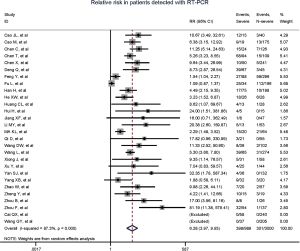
Comparison of myocardial injury risk with severe versus non-severe patients
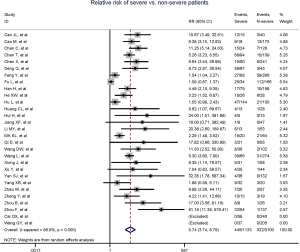
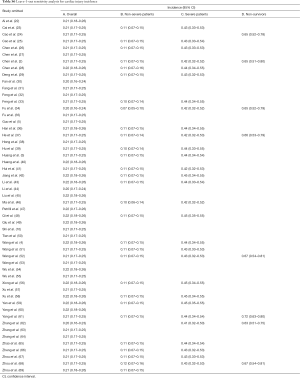
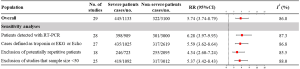
Totally, 29 studies involving 4,233 patients were identified, and the incidence of myocardial injury was 39.3% in severe patients (445/1,133) compared with 10.4% (322/3,100) in non-severe patients (Figure 3), indicating that severe patients were associated with significantly higher risk of myocardial injury (RR, 5.74; 95% CI, 3.74–8.79; I2, 86.8%; Figure S9). Leave-1-out sensitivity analyses as well as further serial sensitivity analyses were in consistence with the primacy results (Figures S10,S11,S12,S13,S14). Meta-regression failed to detect any clinical characteristics and comorbidities to impact the primacy results (Table S7).
Full table
Risk factors associated with myocardial injury
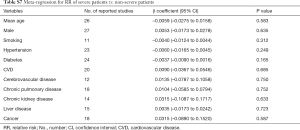
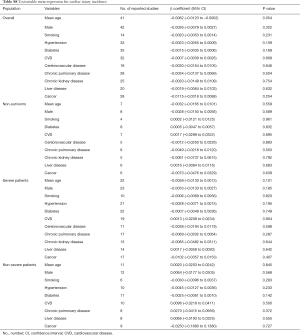
The association between various comorbidities and myocardial injury incidence is shown in Table S8. Eleven variables with more than 25% data points (age, gender, smoking, hypertension, diabetes, CVD, cerebrovascular disease, chronic pulmonary disease, chronic kidney disease, liver disease, and cancer) were assessed in univariable meta-regression. The results suggested that the incidence of myocardial injury were not associated with any of the above comorbidities.
Full table
Publication bias
The funnel plots for myocardial injury incidence in overall patients, in severe patients, and in non-severe patients were all asymmetrical on visual inspection, and the corresponding P values for the Egger’s test were <0.001, 0.972, and 0.004, respectively (Figure S15). The trim and fill method was applied to handle publication bias, resulting in 9% (95% CI, 5–14%) for incidence in overall patients and 4% (95% CI, 1–7%) for incidence in non-severe patients (Table S9). Because of limited study number in non-survivors (8 studies), funnel plot was not performed.
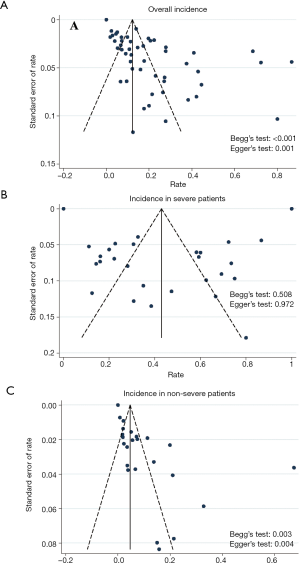
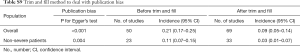
Full table
Discussion
Major findings and interpretations
This systematic review and meta-analysis firstly provided a comprehensive overview of myocardial injury incidence based on 53 retrospective studies involving 7,679 COVID-19 patients. The major findings were as follows: (I) the overall incidence of myocardial injury was 21%; (II) considering the severity of disease, myocardial injury incidence progressively increased in non-survivors (66%), severe patients (43%), and non-severe patients (11%); (III) severe patients had a 4.74-fold increased risk of myocardial injury compared with non-severe patients. Previous study found that COVID-19 patients who were admitted to the ICU had higher plasma levels of cytokines (3). As cytokine storm is one of the potential mechanisms underlying myocardial injury, it is predictable that incidence of myocardial injury might be high among non-survivors and severe patients.
Comparison with previous studies
Currently, several systematic reviews and meta-analyses have been conducted to assess the risk of myocardial injury among COVID-19 patients. The earliest one, which pooled 4 studies of 341 patients, showed that the values of troponin were significantly increased in severe patients than that in non-severe patients [standardized mean difference (SMD), 25.6 ng/L; 95% CI, 6.8–44.5 ng/L] (10). Although this is the first meta-analysis to assess the myocardial injury risk in COVID-19, the limitation of study number and sample size may influence the robustness of results. Another meta-analysis addressed this issue by merging 28 studies of 4,189 patients and found that myocardial injury biomarkers were higher in severe patients compared with less severe patients (SMD, 0.69; 95% CI, 0.48–0.89) (11). Notably, this study used a broadly definition of myocardial injury (the combination of troponin, CK-MB, NT-proBNP, and myoglobin), which inevitably led to the overestimation of myocardial injury risk. The recent meta-analysis involving 2,389 patients from 13 studies reported that myocardial injury was associated with higher mortality (RR, 7.95; 95% CI, 5.12–13.34) and need for ICU care (RR, 7.94; 95% CI, 1.51–41.78) (12). This study used a precise definition of myocardial injury (troponin above the 99th percentile upper reference limit, regardless of new abnormalities in electrocardiography and echocardiography), whereas risk factors of myocardial injury as well as visible publication bias seemingly not to be well addressed. Given the above limitations, the present meta-analysis restricted the definition of myocardial injury and included all available evidence to comprehensively estimate the incidence and potential risk factors of myocardial injury in COVID-19 patients.
Potential mechanism of myocardial injury
The mechanisms underlying myocardial injury have not well established but likely involve viral myocarditis, cytokine storm, microvascular thrombosis, and unmasked CVDs. Evidence from autopsies found that 35% of heart samples in SAR-CoV infected patients presented the viral genome, which raised the possibility of direct impair of cardiomyocytes by the virus (19). SAR-CoV-2 might share the same mechanism as the highly homologous with SAR-CoV. Nevertheless, a recent pathological study failed to demonstrate the presence of SAR-CoV-2 within myocardial tissue (20). Therefore, the question of whether the SAR-CoV-2 could directly damage the heart requires further scientific verification. Guo et al. found that plasma troponin levels had a significantly positive linear correlation with plasma high-sensitivity C-reactive protein (hs-CRP) levels, indicating that myocardial injury may be associated with inflammatory pathogenesis during the disease progress (5). Besides hs-CRP, other cytokines, including interleukin (IL)-2, IL-7, IL-10, IgG-included protein 10, monocyte chemoattractant protein-1, macrophage inflammatory protein 1-alpha, and tumors necrosis factor, were proved to be involved in the inflammatory response of COVID-19 (3). The activation of these inflammatory cytokines after infection might cause endothelial dysfunction, coronary plaque destabilization, microvascular dysfunction, and subsequently contribute to myocardial injury. Predictably, this marked inflammatory response could also lead to the development of disseminated intravascular coagulopathy (DIC) in critical patients. Tang et al. reported that coagulopathy was associated with high mortality and 71% of non-survivors met the criteria of DIC (21). As such, microvascular thrombosis of coronary vessels due to DIC is another potential mechanism that might contribute to myocardial injury. In addition, COVID-19 patients preexisting CVD and other comorbidities might be more likely to suffer from myocardial injury. Shi et al reported that approximately 30% and 60% of patients with myocardial injury had a history of coronary heart disease and hypertension, respectively, which were more prevalent than in those without myocardial injury (6). Although limited evidence exists for evaluating the association of myocardial injury with cardiovascular comorbidities, it is rational to presume that patients with underlying comorbidities are susceptible to myocardial injury through several mechanisms including virus-driven direct damage, systemic inflammatory response, coronary plaque destabilization, and hypoxia aggravation. Regrettably, we only obtained study-level information about comorbidities and failed to detect any risk factors associated with myocardial injury.
Clinical consideration for myocardial injury
Given the high incidence of myocardial injury among COVID-19 patients, it might be reasonable to triage patients according to cardiovascular comorbidities and myocardial biomarkers. The majority of patients with a mildly elevated troponin can be followed with expectant management until recovery from acute viral syndrome. However, patients whom are hemodynamically and electrophysiologically unstable with marked elevations of troponin should launch earlier and more aggressive intervention strategies.
Strengths and limitations
Strengths of this study mainly include the systematic and rigorous approach to estimate the incidence of myocardial injury. We performed a comprehensive search of English-language databases, Chinese-language, and preprint platform; restricted the definition of myocardial injury; used the revised NOS tool to suitably assess the study quality; conducted the subgroup analyses by disease severity to explore for differences on myocardial injury incidence; performed serial sensitivity analyses to strengthen the robustness of results; applied meta-regression to explore the risk factors associated with myocardial injury; and employed trim and fill method to handle the potential publication bias. Certainly, several intrinsic limitations should be recognized in this study. Firstly, all included studies were retrospective and there were differences on diagnosis criterion for COVID-19 and definition of myocardial injury. To account for these issues, we have conducted sensitivity analyses by only including studies that patients were detected with RT-PCR assay or cases were defined as troponin. The results of sensitivity analyses were in line with the primacy results. Secondly, given the difficulty of performing echocardiography or cardiac magnetic resonance imaging under strict isolation, the exact prevalence and nature of myocardial injury in COVID-19 may difficult to be fully illuminating. Thus, in the present study, we used myocardial enzymology indexes as the definition of myocardial injury. Thirdly, we did not obtain patient-level information about comorbidities and concomitant medication for exploring the potential risk factors of myocardial injury. Also, all the included studies did not report the adjusted RRs related to cardiac injury, thus the pooled RRs from crude data may introduce certain bias. Fourthly, there was significant heterogeneity among included studies and the sources of heterogeneity could be partly explained by disease severity. Finally, we did not assess the clinical diagnosis (angina, myocardial infarction, etc.) associated with elevated myocardial enzymes as well as the dynamic change of troponin and the association between myocardial injury and mortality.
Conclusions
This meta-analysis showed that 21% of patients undergoing myocardial injury in the setting of COVID-19. Higher incidence of myocardial injury was observed in non-survivors (66%) and severe patients (43%). Severe patients had a 4.74-fold increased risk of myocardial injury compared to non-severe patients. Aggressive intervention strategy might be considered for COVID-19 patients at high risk of myocardial injury.
Supplementary
Incidence of myocardial injury in COVID-19: a pooled analysis of 7,679 patients from 53 studies.
Acknowledgments
Funding: This study was supported by the Research Funds of Shanghai Health and Family Planning commission (20184Y0022), Cultivation fund of clinical research of Renji Hospital (PY2018-III-06), Clinical Pharmacy Innovation Research Institute of Shanghai Jiao Tong University School of Medicine (CXYJY2019ZD001, CXYJY2019QN004), and Program for Key but Weak Disciplines of Shanghai Municipal Commission of Health and Family Planning (2016ZB0304).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/cdt-20-535
Peer Review File: Available at http://dx.doi.org/10.21037/cdt-20-535
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-535). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The ethical approval and consent are not required because no patient-level data is involved for this systematic review and meta-analysis.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report-147. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200615-covid-19-sitrep-147.pdf?sfvrsn=2497a605_4
- Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020;368:m1091. [Crossref] [PubMed]
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506. [Crossref] [PubMed]
- Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061-9. [Crossref] [PubMed]
- Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:1-8. [Crossref] [PubMed]
- Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;5:802-10. [Crossref] [PubMed]
- Babapoor-Farrokhran S, Gill D, Walker J, et al. Myocardial injury and COVID-19: possible mechanisms. Life Sci 2020;253:117723. [Crossref] [PubMed]
- Hendren NS, Drazner MH, Bozkurt B, et al. Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation 2020;141:1903-14. [Crossref] [PubMed]
- Zhong H, Wang Y, Zhang ZL, et al. Efficacy and safety of current therapeutic options for COVID-19 - lessons to be learnt from SARS and MERS epidemic: a systematic review and meta-analysis. Pharmacol Res 2020;157:104872. [Crossref] [PubMed]
- Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): Evidence from a meta-analysis. Prog Cardiovasc Dis 2020;63:390-1. [Crossref] [PubMed]
- Li JW, Han TW, Woodward M, et al. The impact of 2019 novel coronavirus on heart injury: A systemic review and Meta-analysis. Prog Cardiovasc Dis 2020. [Epub ahead of print]. [Crossref] [PubMed]
- Santoso A, Pranata R, Wibowo A, et al. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: A meta-analysis. Am J Emerg Med 2020. [Epub ahead of print]. [Crossref] [PubMed]
- China National Health Commission. Novel coronavirus diagnosis and treatment plan. seventh ed. 2020. Available online: http://www.gov.cn/zhengce/zhengceku/2020-03/04/content_5486705.htm
- Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj 2011;343:d5928. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Gu ZC, Wei AH, Zhang C, et al. Risk of major gastrointestinal bleeding with new vs conventional oral anticoagulants: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2020;18:792-9.e61. [Crossref] [PubMed]
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1-34. [Crossref] [PubMed]
- Oudit GY, Kassiri Z, Jiang C, et al. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest 2009;39:618-25. [Crossref] [PubMed]
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420-2. [Crossref] [PubMed]
- Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18:844-7. [Crossref] [PubMed]
- Ai J, Chen J, Wang Y, et al. The cross-sectional study of hospitalized coronavirus disease 2019 patients in Xiangyang, Hubei province. medRxiv 2020. doi: 10.1101/2020.02.19.20025023. [Crossref]
- Cai Q, Huang D, Ou P, et al. -nCoV Pneumonia in a Normal Work Infectious Diseases Hospital Besides Hubei Province, China. The Lancet 2019;2020. [Crossref]
- Cao J, Tu WJ, Cheng W, et al. Clinical features and short-term outcomes of 102 patients with corona virus disease 2019 in Wuhan, China. Clin Infect Dis 2020;71:748-55. [Crossref] [PubMed]
- Cao M, Zhang D, Wang Y, et al. Clinical Features of Patients Infected with the 2019 Novel Coronavirus (COVID-19) in Shanghai, China. medRxiv 2020. doi: 10.1101/2020.03.04.20030395. [Crossref]
- Chen C, Chen C, Yan JT, et al. Analysis of myocardial injury in patients with COVID-19 and association between concomitant cardiovascular diseases and severity of COVID-19. Zhonghua Xin Xue Guan Bing Za Zhi 2020;48:E008. [PubMed]
- Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020;130:2620-9. [Crossref] [PubMed]
- Chen X, Zheng F, Qing Y, et al. Epidemiological and clinical features of 291 cases with coronavirus disease 2019 in areas adjacent to Hubei, China: a double-center observational study. medRxiv 2020. doi: 10.1101/2020.03.03.20030353. [Crossref]
- Deng Q, Hu B, Zhang Y, et al. Suspected myocardial injury in patients with COVID-19: evidence from front-line clinical observation in Wuhan, China. Int J Cardiol 2020;311:116-21. [Crossref] [PubMed]
- Chen J, Fan H, Zhang L, et al. Retrospective Analysis of Clinical Features in 101 Death Cases with COVID-19. medRxiv 2020. doi: 10.1101/2020.03.09.20033068. [Crossref]
- Fang X. Clinical characteristics and treatment strategies of 79 patients with COVID-19. Chinese Pharmacological Bulletin 2020;36:453-9.
- Feng T, Yue H, Pu J, et al. Clinical characteristics of several patients with coronavirus disease 2019 in Lanzhou City. Journal of Xi'an Jiaotong University (Medical Sciences) 2020. [Epub ahead of print].
- Feng Y, Ling Y, Bai T, et al. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med 2020;201:1380-8. [Crossref] [PubMed]
- Fu L, Fei J, Xiang H-X, et al. Influence factors of death risk among COVID-19 patients in Wuhan, China: a hospital-based case-cohort study. medRxiv 2020. doi: 10.1101/2020.03.13.20035329. [Crossref]
- Fu S, Fu X, Song Y, et al. Virologic and clinical characteristics for prognosis of severe COVID-19: a retrospective observational study in Wuhan, China. 2 medRxiv 2020. doi: 10.1101/2020.04.03.20051763. [Crossref]
- Han H, Xie L, Liu R, et al. Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan, China. J Med Virol 2020;92:819-23. [Crossref] [PubMed]
- He XW, Lai JS, Cheng J, et al. Impact of complicated myocardial injury on the clinical outcome of severe or critically ill COVID-19 patients. Zhonghua Xin Xue Guan Bing Za Zhi 2020;48:E011. [PubMed]
- Hong Z, Zheng X, Yang X, et al. Comparative analysis of the clinical characteristics of 18 severe/critical coronavirus disease 2019 patients with myocardial injury. Chin J Arterioscler 2020;28:290-5.
- Hu L, Chen S, Fu Y, et al. Risk Factors Associated with Clinical Outcomes in 323 COVID-19 Patients in Wuhan, China. medRxiv 2020. doi: 10.1101/2020.03.25.20037721. [Crossref]
- Huang Y, Tu M, Wang S, et al. Clinical characteristics of laboratory confirmed positive cases of SARS-CoV-2 infection in Wuhan, China: A retrospective single center analysis. Travel Med Infect Dis 2020. [Epub ahead of print]. [Crossref] [PubMed]
- Hui H, Zhang Y, Yang X, et al. Clinical and radiographic features of cardiac injury in patients with 2019 novel coronavirus pneumonia. medRxiv 2020. doi: 10.1101/2020.02.24.20027052. [Crossref]
- Jiang X, Tao J, Wu H, et al. Clinical features and management of severe COVID-19: a retrospective study in Wuxi, Jiangsu Province, China. medRxiv 2020. doi: 10.1101/2020.04.10.20060335. [Crossref]
- Li M, Lyu M, Li C, et al. Analysis on the cardiac features of patients with different clinical types of novel coronavirus disease 2019. Guangdong Medical Journal 2020;8:797-800.
- Li X, Wang L, Yan S, et al. Clinical characteristics of 25 death cases infected with COVID-19 pneumonia: a retrospective review of medical records in a single medical center, Wuhan, China. medRxiv 2020. doi: 10.1101/2020.02.19.20025239. [Crossref]
- Liu Y, Li J, liu D, et al. Clinical features and outcomes of 2019 novel coronavirus-infected patients with cardiac injury. medRxiv 2020. doi: 10.1101/2020.03.11.20030957. [Crossref]
- Ma KL, Liu ZH, Cao CF, et al. COVID-19 myocarditis and severity factors: an adult cohort study. medRxiv 2020. doi: 10.1101/2020.03.19.20034124. [Crossref]
- Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 disease in New York City. medRxiv 2020. doi: 10.1101/2020.04.08.20057794. [Crossref]
- Qi D, Yan X, Tang X, et al. Epidemiological and clinical features of 2019-nCoV acute respiratory disease cases in Chongqing municipality, China: a retrospective, descriptive, multiple-center study. medRxiv 2020. doi: 10.1101/2020.03.01.20029397. [Crossref]
- Qiu C, Xiao Q, Liao X, et al. Transmission and clinical characteristics of coronavirus disease 2019 in 104 outside-Wuhan patients, China. medRxiv 2020. doi: 10.1101/2020.03.04.20026005. [Crossref]
- Tian S, Zhu X, Sun X, et al. Longitudinal analysis of laboratory findings during the process of recovery for patients with COVID-19. medRxiv 2020. doi: 10.1101/2020.04.04.20053280. [Crossref]
- Wang G, Wu C, Zhang Q, et al. Epidemiological and Clinical Features of Corona Virus Disease 2019 (COVID-19) in Changsha, China. The Lancet 2020. [Crossref]
- Wang L, He W, Yu X, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect 2020;80:639-45. [Crossref] [PubMed]
- Wang S, Peng W, Yu L, et al. Clinical analysis of 17 cases of coronavirus disease 2019. Zhejiang Medical Journal 2020;42:365-7.
- Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020;180:1-11. [Crossref] [PubMed]
- Wu J, Liu J, Zhao X, et al. Clinical characteristics of imported cases of COVID-19 in Jiangsu Province: a multicenter descriptive study. Clin Infect Dis 2020;71:706-12. [Crossref] [PubMed]
- Xiong J, Jiang W, Zhou Q, et al. Clinical characteristics, treatment, and prognosis in 89 cases of COVID-2019. Medical Journal of Wuhan University 2020. [Crossref]
- Xu H, Hou K, Xu H, et al. Acute Myocardial Injury of Patients with Coronavirus Disease 2019. medRxiv 2020. doi: 10.1101/2020.03.05.20031591. [Crossref]
- Xu Y, Li Y, Zeng Q, et al. Clinical characteristics of SARS-CoV-2 pneumonia compared to controls in Chinese Han population. medRxiv 2020. doi: 10.1101/2020.03.08.20031658. [Crossref]
- Yan S, Song X, Lin F, et al. Clinical Characteristics of Coronavirus Disease 2019 in Hainan, China. medRxiv 2020. doi: 10.1101/2020.03.19.20038539. [Crossref]
- Yang K, Xiao L, Liu Y, et al. Epidemiological and clinical characteristics of coronavirus disease 2019 in nonepidemic areas: report of 57 cases. Journal of the third military medical university 2020;42:555-9.
- Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8:475-81. [Crossref] [PubMed]
- Zhang B, Zhou X, Qiu Y, et al. Clinical characteristics of 82 death cases with COVID-19. medRxiv 2020. doi: 10.1101/2020.02.26.20028191. [Crossref]
- Zhang F, Yang D, Li J, et al. Myocardial injury is associated with in-hospital mortality of confirmed or suspected COVID-19 in Wuhan, China: A single center retrospective cohort study. medRxiv 2020. doi: 10.1101/2020.03.21.20040121. [Crossref]
- Zhang Q, Yang Z, Li Y, et al. Coronary heart disease and its risk factors in COVID-19 patients with mild symptoms: clinical characteristics summary. Chinese Heart Journal 2020;32:119-23,127.
- Zhao W, Yu S, Zha X, et al. Clinical characteristics and durations of hospitalized patients with COVID-19 in Beijing: a retrospective cohort study. medRxiv 2020. doi: 10.1101/2020.03.13.20035436. [Crossref]
- Zheng Y, Sun L, Xu M, et al. Clinical characteristics of 34 COVID-19 patients admitted to ICU in Hangzhou, China. medRxiv 2020. doi: 10.1101/2020.04.12.20062604. [Crossref]
- Zhou B, She J, Wang Y, et al. The clinical characteristics of myocardial injury in severe and very severe patients with 2019 novel coronavirus disease. J Infect 2020;81:147-78. [Crossref] [PubMed]
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054-62. [Crossref] [PubMed]
- Zhou F, Yu X, Tong X, et al. Clinical features and outcomes of 197 adult discharged patients with COVID-19 in Yichang, Hubei. medRxiv 2020. doi: 10.1101/2020.03.26.20041426. [Crossref]