
Vessel wall MR imaging for the detection of intracranial inflammatory vasculopathies
Introduction
Conventional arterial imaging of intracranial vessels focuses on the analysis of the vessel lumen, enabling to search for vessel irregularities, stenosis, or occlusion on angiography. Although angiography allows detecting abnormal variations of vessel lumen size, it lacks specificity as different pathologies produce similar luminal defects. Moreover, some disorders of the intracranial arteries such as intracranial atherosclerotic disease (ICAD) or vasculitis arise within the vessel wall, without always narrowing the lumen or modifying the regularity of the lumen, particularly at the initial phase.
Thanks to MR sequences designed to provide high spatial resolution with a black blood effect, it has become possible to image the vessel wall in 3 dimensions and to detect pathological changes in the wall. An early application was reported for vasculitis, where wall thickening and intramural contrast uptake were described in patients with active vasculitis affecting large brain arteries (1). Development of 3D high-resolution black blood sequences for intracranial vessel wall MR imaging (VW-MRI) enabled routine clinical applications not only vasculitis, but also of ICAD, intracranial dissections, reversible intracranial dissections, reversible cerebral vasoconstriction syndrome (RCVS), Moyamoya disease, and intracranial aneurysms. There is also potential to use VW-MRI to follow disease progression and monitor treatment effects such as for vasculitis or ICAD. In this article, we review the imaging principles and pitfalls of VW-MRI, and recommend applications for vascular diseases including non-occlusive intracranial vasculopathies, Moyamoya disease, and identifying culprit plaques. We review the utility of VW-MRI for determining stroke etiology in adults with a separate focus on stroke in children and young adults.
Imaging principles and pitfalls
This high-resolution intracranial VW- MRI approach is increasingly used on a clinical basis at many centers to solve diagnostic problems, especially in patients with ischemic stroke or intracranial hemorrhage. An expert consensus Guideline (2) from the American Society of Neuroradiology provides recommendations for clinical implementation of intracranial vessel wall MRI. There are several technical aspects needed to be considered when implementing VW-MRI in intracranial vessels, including flow suppression, both in blood and cerebrospinal fluid (CSF), spatial resolution and signal-to-noise ratio (SNR).
The primary mechanism for blood suppression effects of 3D VW-MRI is motion-induced intra-voxel dephasing (3). Blood with a wide range of velocities flowing across a magnetic field leads to the phase dispersion and signal loss. The blood suppression effect is enhanced by the use of low flip-angle refocusing pulses enabling long echo trains that promotes larger phase dispersion, especially when the flow direction is along with the frequency encoding axis (4). Additionally, the variable-flip-angle refocusing pulses introduce the formation of simulated echoes, which exhibits a complicated phase evolution leading to wide phase dispersion and strong blood suppression effect.
In general, the CSF-signal suppression effect can be attained by tailoring image contrast to T1 weighting due to its long T1 value. Implementation of an inversion recovery pre-pulse could theoretically null the CSF signal but may suffer from SNR deficiency, which is insufficient to resolve the intracranial vessel wall at 3T (3). Although DANTE-prepared VW-MRI can suppress the signal of CSF to some extent, the effect is diminished when CSF flow is below 0.1 cm/s, which is particularly problematic at CSF regions surrounding the middle cerebral arteries (5). Incorporation of a flip-down radiofrequency pulse module (e.g., anti-drive) to 3D VW-MRI has achieved enhanced CSF-signal suppression (6,7). It employs a positive 90° radiofrequency pulse at the end of the echo train to tip the transverse magnetization into the negative longitudinal plane, thereby, suppresses the transverse magnetization and minimizes signals of tissues with long T2 values (e.g., CSF).
Given the small size of intracranial vessels (normal wall thickness ranges 0.3–0.6 mm, VWI-ASNR study group has recommended an acquired resolution of 0.5 mm isotropic as a starting point for intracranial VW-MRI (2). Higher magnetic field strength (i.e., 7-T) offers the potential to increase the spatial resolution of intracranial VW-MRI, but this potential has not yet been realized (8). The 3D VW-MRI technique demonstrates high SNR-efficiency, providing a 58% improvement in SNR of vessel wall compared with 2D MRI sequence (3). The employed variable-flip-angle refocusing pulses allow for a longer echo train length without compromising signal within a relatively short scan time.
3D VW-MRI has gained popularity in the diagnosis and management of patients with cerebrovascular disease in clinical practice. The technique can, however, generate artifacts that arise from the technical caveats (e.g., slow or recirculated flow or inadequate spatial resolution). Blood flow near the vessel wall may not be fully suppressed as the flow is often slower compared with the center of the lumen, which can cause artificial wall thickening. The slow flow in small veins can be misinterpreted as arterial wall enhancement after contrast injection. The recirculating or disturbed flow may yield plaque-mimicking flow artifacts due to incomplete flow suppression, which commonly seen in the curved and large vessel segments. Contrast-enhanced MRA is often helpful in excluding flow-related artifacts. In addition, blood suppression is more effective when the flow direction is along with the frequency encoding axis (4). Acquiring VW-MRI in multiple orientations may help to discern the artifacts caused by orientation-related incomplete flow suppression.
Inadequate spatial resolution can induce partial volume averaging. This can lead to the overestimation (9) of the wall thickness and could be misinterpreted as atherosclerotic plaque or vasculitis, in particular, with corresponding enhancement seen on contrast-enhanced VW-MRI.
Detection of non-occlusive intracranial vasculopathies
Historically, angiographic imaging has served as the reference standard for the differentiation and characterization of non-occlusive vasculopathies. Intracranial vasculopathies are routinely investigated by lumen-based modalities such as magnetic resonance angiography (MRA), computed tomography angiography (CTA), and digital subtraction angiography (DSA). These techniques are useful to analyze the vessel lumen, allowing to detect vessel stenosis or occlusion. However, the primum movins of the disease, i.e., an abnormal thickening of the vessel wall, remains within the arterial wall. Therefore these modalities, focusing on lumen, reflect an indirect marker of an abnormality of the vessel wall, and may be insufficient in detecting lesions that do not restrict the lumen (10). Wall thickening and intramural contrast uptake have been described as characteristic findings in patients with active cerebral vasculitis affecting large brain arteries (11,12). Several VW-MRI studies have reported that intracranial atherosclerotic lesions can also enhance on VW-MRI and the enhancement is associated with increased risk of stroke events (13-15). Therefore, lesions of different etiologies, modifying the arterial wall structure, may be detected using VW-MRI on non-contrast exams (hematoma and intracranial dissection) and after gadolinium administration (atherosclerosis, vasculitis, angeitis…)
Furthermore, VW-MRI, combined with MR Angiography in the evaluation of suspected vasculopathies, has been shown to identify a higher number of abnormal vessel segments in comparison with 3D-TOF-MRA alone (11,13,16). This may be useful both for the detection and characterization of the intracranial arteriopathy. VW-MRI in addition to MRA significantly improves diagnostic accuracy for both per-lesion and per-patient analyses when compared to luminal imaging alone (per-lesion: 88.8% vs. 36.1%, P<0.001, per-patient: 96.3% vs. 43.5%, P<0.001), respectively (11). Song et al. (16) confirmed this hypothesis on another cohort of ≈100 patients.
Characterization of non-occlusive vasculopathy
Besides the importance of the detection and the characterization of the extension of an intracranial vasculopathy, different patterns of enhancement have been described for the diagnosis and subgroup differentiation of intracranial vasculopathies.
Pattern and distribution are the two main features of enhancement that can vary depending on the etiology. The pattern may be concentric, eccentric, or focal and nodular. The distribution may also be solitary or multifocal. MRA abnormalities can be found in the presence of abnormal vessel wall enhancement with either a decrease in size of the outer diameter at the lesion site (negative remodeling) or with a normal to increase in diameter (positive remodeling) (17,18).
Atherosclerotic plaque
Histopathological studies of the composition of plaques offer strong evidence that intraplaque hemorrhage and inflammation are significant risk factors for ischemic symptoms, regardless of stenosis severity (17).
This evidence highlights the role of plaque imaging and plaque phenotyping in risk stratification and clinical decision making in addition to stenosis parameters, and have been mainly validated with histopathological evidence from resected atherosclerotic lesions of the bulbar carotid artery.
However, although thinner, intracranial vessels have already been studied with post gadolinium VW MRI in more than 20 studies and several meta-analyses (13,14,19,20) focused on the determination of markers of intracranial culprit plaque. These studies typically exclude other causes of stroke, such as atrial fibrillation, severe cervical carotid stenosis, or vasculitis. The main VW MRI findings of ICAD plaque are eccentric enhancement of the plaque with a variable length of involvement remaining most frequently focal (11,15,21). In patients with recent stroke, multiple studies have reported an association between T1 post-contrast plaque enhancement and the symptomatic status of the ICAD plaque (Figure 1).
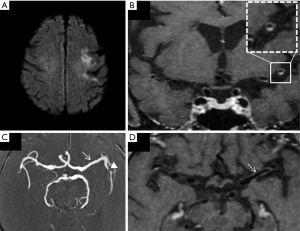
Lee et al. (14) reported that intracranial plaque with contrast enhancement, positive remodeling, and wall irregularity were significantly more likely to be associated with ischemic stroke at the corresponding territory. On a meta-analysis, using a random effects model, Gupta et al. (19) reported that infarction was 10 times more likely in tissue supplied by an enhancing artery than in tissue supplied by nonenhancing arteries. Overall in their study, 59% of the culprit plaques were enhancing versus 23% of the non-culprit plaques.
Several studies noted that the intensity of enhancement was different when comparing enhancing plaques, leading to or qualitative or quantitative evaluation of the enhancement. Qiao et al. (18) proposed to test an ordinal scale with three levels of enhancement of the ICAD plaque compared to that of the pituitary infundibulum. She concluded that the higher the grade, the higher is the probability of the plaque to be the culprit for stroke.
Quantitative analysis (22) of the enhancement, comparing pre and post-contrast intensity values of the ICAD plaque, support this hypothesis and found that the percentage increase in enhancement after contrast was significantly higher for symptomatic plaques (63 vs. 23%, P=0.001). An analysis of different plaque features found that high signal on T1-weighted images, wall thickening pattern, and a high degree of postcontrast enhancement were associated with culprit plaque (23).
As suggested by a recent study (24), the wall enhancement in ICAD lesions is variable over time. Contrast enhancement of ICAD can persist months after the ischemic event. Preliminary results (21) suggest that follow-up improves accuracy in identifying culprit plaques. Lack of enhancement at baseline or a decrease in enhancement at follow-up may suggest that the plaque is not the culprit.
Systemic vasculitis and primary angiitis of the central nervous system
Cerebral vasculitis is defined as inflammation of arterial walls leading to progressive obstruction of the lumen, increased coagulation, and hence risk of stroke. Primary angiitis of the central nervous system (25) can occur as an idiopathic disorder restricted to the CNS, but most often, vasculitis is associated with systemic connective tissue disorders or may be secondary to non-inflammatory process: infection, neoplasm, drugs, or radiation therapy (26,27).
The 2012 Chapel Hill Consensus Conference categorized vasculitis in terms of size of the involved arteries (small, medium, or large vessels) and associated pathologic lesions (27).
The angiographic diagnosis of vasculitis depends on the presence of one or multiple stenoses of brain vessels. Straightening and kinking of arteries can be seen as consequences of the induration and stiffening of the vascular walls.
The MR imaging diagnosis relies on both the detection of vascular stenosis by 3D time of flight MRA and circumferential enhancement on VW-MRI (28). The enhancement can be either pencil-thin or thick, extending beyond the margin of the vessel wall (11,12). One or multiple stenoses can be identified, and their detection can be sensitized by the presence of circumferential enhancement on VW-MRI. The ability of vascular MR imaging to identify vasculitis relates to the size of the affected vessels (29).
Proximal involvement has been observed (30) in sarcoid, giant cell arteritis (GCA), systemic sclerosis, primary angiitis of the central nervous system, and infectious disease, foremost among which is Varicella Zoster vasculitis (Figure 2), frequently affecting M1 segment, responsible of basal ganglia infarcts. This can occur after primary or reactivated Varicella Zoster virus infection and in patients who are immunocompetent or immunosuppressed (31). Other infectious vasculitis etiologies are bacterial meningitis by pneumococcus and meningococcus, tuberculosis, Treponema pallidum, Borrelia burgdorferi, and fungus (27).
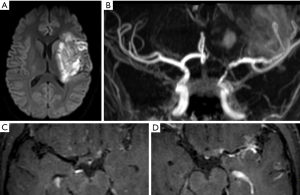
In GCA, VW MRI allows to analyze intracranial arteries (Figure 3) and external branches of the carotid artery, such as the superficial temporal artery, looking for circumferential enhancement. Interestingly, Rhéaume et al. (32) reported in a correlation of imaging and histopathological findings in patients with suspected GCA, that normal findings on scalp artery MRI are very strongly associated with negative temporal artery biopsy findings. This finding suggests that MRI could be used as the initial diagnostic procedure in GCA, with temporal artery biopsy being reserved for patients with abnormal MRI findings. Zeiler et al. (12) showed the post-contrast 3D high resolution VW-MRI can be used to identify inflamed intracranial vascular targets for biopsy, greatly improving the diagnostic accuracy of biopsies for CNSV.
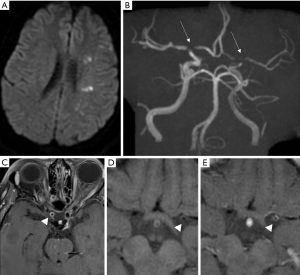
Vasculitis affecting medium-sized arteries are caused by a wide spectrum of disorders (30), such as panarteriitis nodosa (33), lupus erythematosus (34), Behcet’s disease (35). Although VW-MRI is useful to detect abnormal enhancing segments in vasculitis, it cannot however distinguish between these disorders (12).
Detecting wall thickening and vessel wall enhancement may also serve as biomarkers of treatment efficacy. However, as reported by Reichenbach et al. on a controlled trial of GCA patients under tocilizumab (36), vessel wall enhancement normalized in only one-third of patients yet presenting a complete clinical and laboratory remission. Hence, whether these signals are of prognostic importance remains to be determined.
RCVS
Recurrent thunderclap headache is the most common symptom of patients with RCVS, for which the main differential diagnosis is subarachnoid hemorrhage. RCVS is supposedly due to a transient disturbance in the control of cerebrovascular tone and often resolves spontaneously within 3 months. More than half the cases occur postpartum or after exposure to adrenergic or serotonergic drugs. It can be difficult to distinguish RCVS from other arteriopathies since most lack a gold standard diagnostic test and are similarly associated with headache and stroke. Early discrimination is important because of management differences: in RCVS, CSF examination and brain biopsy have little utility other than to exclude mimics. Although the prototypical demographics and classic presentation of RCVS (most commonly seen in young or middle-aged females with numerous described risk factors) differ from CNS vasculitis, diagnostic uncertainty may remain after conventional luminal imaging in some cases. It is important to differentiate between the two diagnoses because RCVS does not require anti-inflammatory or immunosuppressive treatment. As a non-inflammatory disease, RCVS has been described as presenting with non enhancing concentric wall thickening (11,37) corresponding to the areas of multifocal segmental arterial narrowing. However some cases have also been reported as presenting marked enhancement, highlighting the limited specificity of lack of enhancement in RCVS (11).
Moya-moya vasculopathy
Moyamoya syndrome is a vasculopathy exclusive to the vasculature of the brain that results in significant stenosis of the proximal intracranial vessels. This abnormality results in decreased flow to the brain and is associated with an increased risk of ischemic and hemorrhagic stroke. In an effort to compensate for lack of flow, collateral vessels develop near the internal carotid artery apex, cortical apex, and brain perforating vessels in the basal ganglia.
The syndrome occurs in a bimodal chronology and is common in children who are approximately five years of age and adults 40–50 years (38-40). The reported incidence -rate ratios is 4.6 for Asian Americans, 2.2 for blacks, and 0.5 for Hispanics, as compared with whites (41).
While ischemic events are common in children, hemorrhagic is common in adults. Ischemia is commonly associated with areas of border zone circulation related to ICA stenosis and MCA stenosis. Hemorrhage, on the other hand, occurs 10–40% of cases and is frequent in the basal ganglia with intraventricular extension, or subarachnoid hemorrhage.
The pathophysiology of Moyamoya has always been intriguing for neuropathologists, it is a non-inflammatory and non-atheromatous disease of the intracranial circulation that shows fibrocelluar thickening of the wall, irregular undulation of the internal elastic laminae, medial thinness and a decrease in the outer diameter (41-43).
Approximately 10% of individuals with MMD exhibit a familial occurrence and is associated with other genetically related diseases such as collagen disorders, Down syndrome, neurofibromatosis, and sickle cell (44).
The diagnosis of Moyamoya relies heavily on symptoms and imaging characteristics. CT imaging could demonstrate border zone infarcts, hypodensities in the basal ganglia, or be entirely normal in some patients. CT angiography may demonstrate the classical vessel characteristics of narrowing of the supraclinoid segment of the ICA, proximal, anterior cerebral arteries, middle cerebral arteries, and later proliferation of collateral vessels. The Suzuki grading system describes the stages of Moyamoya (45).
MR angiography is a very sensitive technique that besides demonstrating acute strokes, also demonstrates multiple abnormalities of the intracranial circulation of patients with Moyamoya syndrome, and may provide brain perfusion data that can guide a surgeon’s approach. Newer MRI technology, such as VW-MRI, has identified unknown features of the disease in its different phases.
A multi-contrast vessel-wall MRI protocol with luminal imaging could help differentiate between moyamoya disease, atherosclerotic-moyamoya syndrome and vasculitic-moyamoya syndrome significantly better than luminal imaging alone (46). It has also been reported that Moyamoya disease may also have different presentations depending on the phase of the disease. The most common pattern to be identified has been concentric enhancement on bilateral distal internal carotid arteries and shrinkage of a middle cerebral artery, regardless of symptoms (47). The same group reported that eccentric enhancement corresponded with acutely infarcted territories, which is a link to vulnerable lesions in Moyamoya disease (Figure 4). There is also evidence of lower vessel wall cross-sectional area in patients with Moyamoya disease compared to vessels of patients with ICAD.
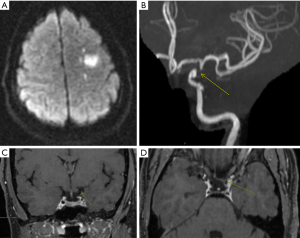
Applications for determining stroke etiology
In adults
As we have shown, patterns and intensity of post gadolinium enhancement of vascular lesions using postcontrast VW-MRI have been used to differentiate between intracranial vasculopathies. Its application to detect the cause of stroke is therefore promising, especially in cryptogenic stroke, for which no cause of stroke has been detected. Indeed, in at least 30% of cases, the exact etiology of stroke cannot be determined using existing investigative protocols (48). Adding VW-MRI may therefore help to detect etiologies that cannot be diagnosed using only luminal angiograms. In the adult stroke population, several studies have already tested the utility of intracranial high-resolution VW MRI in differentiating intracranial vasculopathic diseases causing stroke (49-52). Yet, the diagnostic utility of VW-MRI in the work-up of ischemic stroke still remains a subject of debate, namely on its impact regarding patient outcomes (53). Kesav et al. (49) reported a large number of stroke etiology reclassification due to the use of VW-MRI in cases originally classified as “undetermined” etiologies. In this cohort, compared to the TOAST classification, VW-MRI reclassified 5/49 patients from undetermined etiology to determined etiology, mainly by diagnosing ICAD plaques. This was reinforced in a larger cohort of 205 patients (50), in which 116/205 patients were reclassified as presenting an ICAD plaque thanks to the analysis of VW-MRI. Hence, ICAD is under diagnosed when only analyzing the lumen (54). ICAD might therefore be the most frequent underdiagnosed etiology of stroke in the cryptogenic stroke population. An important question would be to know the temporal course of enhancement of ICAD and whether enhancement would sign a link depending on the delay from stroke symptoms onset. Kwee et al. (21) reported that follow up could help improving accuracy in identifying culprit plaques, as they present enhancement that persists during follow-up, whereas non culprit plaque or do not enhance at baseline or present a decrease in enhancement during follow up. Beside stroke related ICAD diagnosis, VW-MRI can highlight previously described pattern of lesions suggesting underlying causes such as vasculitis or RCVS (11).
The specificity of VWI in children and young adults with stroke
Ischemic and hemorrhagic stroke represent a significant burden in the pediatric population, with long-term medical and psychosocial consequences (55,56). In this age group, vascular imaging plays a central role in the diagnosis and etiologic classification of underlying processes that largely differ from those found in the adult population (57).
Arteriopathy, for instance, is amongst the predominant causes of arterial ischemic strokes in children and may include Moya Moya Disease or Moya Moya syndromes, focal cerebral arteriopathy (FCA) of childhood or post-varicella arteriopathy, intracranial arterial dissection, and less commonly primary angiitis of the central nervous system. As these arteriopathies may present acutely, or at early stages, with overlapping or quasi-similar radiological aspects (58-60), they are very difficult to discriminate from each other using purely luminal imaging techniques (59). Collaterals, either from deep perforators or from leptomeningeal arteries, are known to be an important discriminant but bear imperfect diagnostic and prognostic capacities. Yet, it is critical to correctly classify the underlying processes, which may have dissimilar ischemic recurrence rates, mid-long term evolution, and be approached with different treatment strategies.
Still, the role of VW-MRI in childhood arteriopathies remains poorly understood, as data only begin to be reported. Recently, Stence et al. categorized vessel wall enhancement on a 3 grade scale (none, mild, strong) at the site of luminal abnormalities detected using 3T MRA in a cohort of 16 children with acute ischemic stroke (61). They reported that strong vessel enhancement at baseline was associated with arteriopathy progression, while no enhancement was associated with milder evolutions. Of important note, they also showed that patients treated with steroid courses were more likely to have decreased enhancement at follow-up. Two recent studies (62,63) aimed to explore the clinical characteristics and diagnostic utility of VW-MRI in children with acute ischemic stroke and Pediatric Focal Cerebral Arteriopathy-Inflammatory Subtype. The authors did not find associations between specific patterns of enhancement and underlying etiologies. In this report, there was a tendency for children with VW enhancement to be older, but possibly reflecting technicalities in the acquisition. As such, those studies highlight the potential role of VW-MRI imaging in care intensity stratification for childhood intracranial arteriopathies, and its importance as a longitudinal biomarker during follow up under anti-inflammatory or etiology-specific treatments. But further work is needed to assess the utility of vessel wall imaging in pediatric arterial ischemic stroke and arteriopathies.
Indeed, there remains a considerable knowledge gap to fill before VW-MRI may inform disease targeted strategies, prognostication, and ultimately influence the outcome.
Conclusions
Vessel wall imaging has provided information beyond the epiphenomena of luminal narrowing in the evaluation of intracranial arteriopathies. As described in this review article, VW-MRI is a noninvasive imaging method that has proved to be useful in the detection of inflammatory changes of the intracranial arteries and veins that are frequent in certain arteriopathies. This information is undoubtedly useful for clinicians and stroke neurologists in the workup of patients with acute stroke. Further work is necessary for many areas; these include the use of VW-MRI as a biomarker for immunosuppressive therapy response, prediction of aneurysmal rupture based on aneurysmal wall enhancement, the clinical use of VW-MRI in the pre-stenotic phase of atheromatous disease, increased resolution, and faster sequences are also needed for our acutely ill patients. Radiologist should be knowledgeable of the normal patterns and pitfalls of vessel wall enhancement and the main morphological characteristics of vessel wall lesions.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Luca Saba) for the series “Advanced Imaging in the diagnosis of Cardiovascular Diseases” published in Cardiovascular Diagnosis and Therapy. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-324). The series “Advanced Imaging in The Diagnosis of Cardiovascular Diseases” was commissioned by the editorial office without any funding or sponsorship. LS served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Cardiovascular Diagnosis and Therapy from Jul 2019 to Jun 2021. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Küker W, Gaertner S, Nagele T, et al. Vessel wall contrast enhancement: a diagnostic sign of cerebral vasculitis. Cerebrovasc Dis 2008;26:23-9. [Crossref] [PubMed]
- Mandell DM, Mossa-Basha M, Qiao Y, et al. Intracranial Vessel Wall MRI: Principles and Expert Consensus Recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol 2017;38:218-29. [Crossref] [PubMed]
- Qiao Y, Steinman DA, Qin Q, et al. Intracranial arterial wall imaging using three-dimensional high isotropic resolution black blood MRI at 3.0 Tesla. J Magn Reson Imaging 2011;34:22-30. [Crossref] [PubMed]
- Jara H, Yu BC, Caruthers SD, et al. Voxel sensitivity function description of flow-induced signal loss in MR imaging: implications for black-blood MR angiography with turbo spin-echo sequences. Magn Reson Med 1999;41:575-90. [Crossref] [PubMed]
- Wang J, Helle M, Zhou Z, et al. Joint blood and cerebrospinal fluid suppression for intracranial vessel wall MRI. Magn Reson Med 2016;75:831-8. [Crossref] [PubMed]
- Fan Z, Yang Q, Deng Z, et al. Whole-brain intracranial vessel wall imaging at 3 Tesla using cerebrospinal fluid-attenuated T1-weighted 3D turbo spin echo. Magn Reson Med 2017;77:1142-50. [Crossref] [PubMed]
- Yang H, Zhang X, Qin Q, et al. Improved cerebrospinal fluid suppression for intracranial vessel wall MRI. J Magn Reson Imaging 2016;44:665-72. [Crossref] [PubMed]
- van der Kolk AG, Zwanenburg JJM, Brundel M, et al. Intracranial vessel wall imaging at 7.0-T MRI. Stroke 2011;42:2478-84. [Crossref] [PubMed]
- Antiga L, Wasserman BA, Steinman DA. On the overestimation of early wall thickening at the carotid bulb by black blood MRI, with implications for coronary and vulnerable plaque imaging. Magn Reson Med 2008;60:1020-8. [Crossref] [PubMed]
- Lin A, Rawal S, Agid R, et al. Cerebrovascular Imaging: Which Test is Best? Neurosurgery 2018;83:5-18. [Crossref] [PubMed]
- Mossa-Basha M, Shibata DK, Hallam DK, et al. Added Value of Vessel Wall Magnetic Resonance Imaging for Differentiation of Nonocclusive Intracranial Vasculopathies. Stroke 2017;48:3026-33. [Crossref] [PubMed]
- Zeiler SR, Qiao Y, Pardo CA, et al. Vessel Wall MRI for Targeting Biopsies of Intracranial Vasculitis. AJNR Am J Neuroradiol 2018;39:2034-6. [Crossref] [PubMed]
- de Havenon A, Mossa-Basha M, Shah L, et al. High-resolution vessel wall MRI for the evaluation of intracranial atherosclerotic disease. Neuroradiology 2017;59:1193-202. [Crossref] [PubMed]
- Lee HN, Ryu C-W, Yun SJ. Vessel-Wall Magnetic Resonance Imaging of Intracranial Atherosclerotic Plaque and Ischemic Stroke: A Systematic Review and Meta-Analysis. Front Neurol 2018;9:1032. [Crossref] [PubMed]
- Qiao Y, Zeiler SR, Mirbagheri S, et al. Intracranial plaque enhancement in patients with cerebrovascular events on high-spatial-resolution MR images. Radiology 2014;271:534-42. [Crossref] [PubMed]
- Song JW, Obusez EC, Raymond SB, et al. Vessel Wall MRI Added to MR Angiography in the Evaluation of Suspected Vasculopathies. J Neuroimaging 2019;29:454-7. [Crossref] [PubMed]
- Finn AV, Nakano M, Narula J, et al. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol 2010;30:1282-92. [Crossref] [PubMed]
- Qiao Y, Anwar Z, Intrapiromkul J, et al. Patterns and Implications of Intracranial Arterial Remodeling in Stroke Patients. Stroke 2016;47:434-40. [Crossref] [PubMed]
- Gupta A, Baradaran H, Al-Dasuqi K, et al. Gadolinium Enhancement in Intracranial Atherosclerotic Plaque and Ischemic Stroke: A Systematic Review and Meta-Analysis. J Am Heart Assoc 2016;5:e003816. [Crossref] [PubMed]
- Wang Y, Liu X, Wu X, et al. Culprit intracranial plaque without substantial stenosis in acute ischemic stroke on vessel wall MRI: A systematic review. Atherosclerosis 2019;287:112-21. [Crossref] [PubMed]
- Kwee RM, Qiao Y, Liu L, et al. Temporal course and implications of intracranial atherosclerotic plaque enhancement on high-resolution vessel wall MRI. Neuroradiology 2019;61:651-7. [Crossref] [PubMed]
- Vakil P, Elmokadem AH, Syed FH, et al. Quantifying Intracranial Plaque Permeability with Dynamic Contrast-Enhanced MRI: A Pilot Study. AJNR Am J Neuroradiol 2017;38:243-9. [Crossref] [PubMed]
- Wu F, Ma Q, Song H, et al. Differential Features of Culprit Intracranial Atherosclerotic Lesions: A Whole-Brain Vessel Wall Imaging Study in Patients With Acute Ischemic Stroke. J Am Heart Assoc 2018;7:e009705. [Crossref] [PubMed]
- de Havenon A, Muhina HJ, Parker DL, et al. Effect of Time Elapsed since Gadolinium Administration on Atherosclerotic Plaque Enhancement in Clinical Vessel Wall MR Imaging Studies. AJNR Am J Neuroradiol 2019;40:1709-11. [PubMed]
- Salvarani C, Brown RD Jr, Christianson TJ, et al. Adult primary central nervous system vasculitis treatment and course: analysis of one hundred sixty-three patients. Arthritis Rheumatol 2015;67:1637-45. [Crossref] [PubMed]
- Abdel Razek AAK, Alvarez H, Bagg S, et al. Imaging spectrum of CNS vasculitis. Radiographics 2014;34:873-94. [Crossref] [PubMed]
- Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013;65:1-11. [Crossref] [PubMed]
- Swartz RH, Bhuta SS, Farb RI, et al. Intracranial arterial wall imaging using high-resolution 3-tesla contrast-enhanced MRI. Neurology 2009;72:627-34. [Crossref] [PubMed]
- Thaler C, Kaufmann-Bühler A-K, Gansukh T, et al. Neuroradiologic Characteristics of Primary Angiitis of the Central Nervous System According to the Affected Vessel Size. Clin Neuroradiol 2019;29:37-44. [Crossref] [PubMed]
- Küker W. Imaging of cerebral vasculitis. Int J Stroke 2007;2:184-90. [Crossref] [PubMed]
- Gilden D, Cohrs RJ, Mahalingam R, et al. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol 2009;8:731-40. [Crossref] [PubMed]
- Rhéaume M, Rebello R, Pagnoux C, et al. High-Resolution Magnetic Resonance Imaging of Scalp Arteries for the Diagnosis of Giant Cell Arteritis: Results of a Prospective Cohort Study. Arthritis Rheumatol 2017;69:161-8. [Crossref] [PubMed]
- Valeyrie L, Bachot N, Roujeau JC, et al. Neurological manifestations of polyarteritis nodosa associated with the antiphospholipid syndrome. Ann Med Interne (Paris) 2003;154:479-82. [PubMed]
- Ide S, Kakeda S, Miyata M, et al. Intracranial vessel wall lesions in patients with systematic lupus erythematosus. J Magn Reson Imaging 2018;48:1237-46. [Crossref] [PubMed]
- Calamia KT, Schirmer M, Melikoglu M. Major vessel involvement in Behçet’s disease: an update. Curr Opin Rheumatol 2011;23:24-31. [Crossref] [PubMed]
- Reichenbach S, Adler S, Bonel H, et al. Magnetic resonance angiography in giant cell arteritis: results of a randomized controlled trial of tocilizumab in giant cell arteritis. Rheumatology (Oxford) 2018;57:982-6. [Crossref] [PubMed]
- Soun JE, Song JW, Romero JM, et al. Central Nervous System Vasculopathies. Radiol Clin North Am 2019;57:1117-31. [Crossref] [PubMed]
- Han DH, Nam DH, Oh CW. Moyamoya disease in adults: characteristics of clinical presentation and outcome after encephalo-duro-arterio-synangiosis. Clin Neurol Neurosurg 1997;99 Suppl 2:S151-5. [Crossref] [PubMed]
- Wakai K, Tamakoshi A, Ikezaki K, et al. Epidemiological features of moyamoya disease in Japan: findings from a nationwide survey. Clin Neurol Neurosurg 1997;99 Suppl 2:S1-5. [Crossref] [PubMed]
- Baba T, Houkin K, Kuroda S. Novel epidemiological features of moyamoya disease. J Neurol Neurosurg Psychiatry 2008;79:900-4. [Crossref] [PubMed]
- Uchino K, Johnston SC, Becker KJ, et al. Moyamoya disease in Washington State and California. Neurology 2005;65:956-8. [Crossref] [PubMed]
- Takagi Y, Kikuta K, Nozaki K, et al. Histological features of middle cerebral arteries from patients treated for Moyamoya disease. Neurol Med Chir (Tokyo) 2007;47:1-4. [Crossref] [PubMed]
- Chmelova J, Kolar Z, Prochazka V, et al. Moyamoya disease is associated with endothelial activity detected by anti-nestin antibody. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2010;154:159-62. [Crossref] [PubMed]
- Ikeda H, Sasaki T, Yoshimoto T, et al. Mapping of a familial moyamoya disease gene to chromosome 3p24.2-p26. Am J Hum Genet 1999;64:533-7. [Crossref] [PubMed]
- Suzuki J, Takaku A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol 1969;20:288-99. [Crossref] [PubMed]
- Mossa-Basha M, de Havenon A, Becker KJ, et al. Added Value of Vessel Wall Magnetic Resonance Imaging in the Differentiation of Moyamoya Vasculopathies in a Non-Asian Cohort. Stroke 2016;47:1782-8. [Crossref] [PubMed]
- Ryoo S, Cha J, Kim SJ, et al. High-resolution magnetic resonance wall imaging findings of Moyamoya disease. Stroke 2014;45:2457-60. [Crossref] [PubMed]
- Bray EP, McMahon NE, Bangee M, et al. Etiologic workup in cases of cryptogenic stroke: protocol for a systematic review and comparison of international clinical practice guidelines. Syst Rev 2019;8:331. [Crossref] [PubMed]
- Kesav P, Krishnavadana B, Kesavadas C, et al. Utility of intracranial high-resolution vessel wall magnetic resonance imaging in differentiating intracranial vasculopathic diseases causing ischemic stroke. Neuroradiology 2019;61:389-96. [Crossref] [PubMed]
- Schaafsma JD, Rawal S, Coutinho JM, et al. Diagnostic Impact of Intracranial Vessel Wall MRI in 205 Patients with Ischemic Stroke or TIA. AJNR Am J Neuroradiol 2019;40:1701-6. [PubMed]
- Schaafsma JD, Mikulis DJ, Mandell DM. Intracranial Vessel Wall MRI: An Emerging Technique With a Multitude of Uses. Top Magn Reson Imaging 2016;25:41-7. [PubMed]
- Mossa-Basha M, Hwang WD, De Havenon A, et al. Multicontrast high-resolution vessel wall magnetic resonance imaging and its value in differentiating intracranial vasculopathic processes. Stroke 2015;46:1567-73. [Crossref] [PubMed]
- Song JW. Impact of Vessel Wall MR Imaging in the Work-Up for Ischemic Stroke. Am J Neuroradiol 2019;40:1707-8. [PubMed]
- Qiao Y, Suri FK, Zhang Y, et al. Racial Differences in Prevalence and Risk for Intracranial Atherosclerosis in a US Community-Based Population. JAMA Cardiol 2017;2:1341-8. [Crossref] [PubMed]
- Ganesan V, Prengler M, McShane MA, et al. Investigation of risk factors in children with arterial ischemic stroke. Ann Neurol 2003;53:167-73. [Crossref] [PubMed]
- Boulouis G, Blauwblomme T, Hak JF, et al. Nontraumatic Pediatric Intracerebral Hemorrhage. Stroke 2019;50:3654-661. [Crossref] [PubMed]
- Bernard T.J, Manco-Johnson M.J, Lo W, et al. Towards a Consensus-Based Classification of Childhood Arterial Ischemic Stroke. Stroke 2012;43:371-7. [Crossref] [PubMed]
- Dlamini N, Freeman JL, Mackay MT, et al. Intracranial dissection mimicking transient cerebral arteriopathy in childhood arterial ischemic stroke. J Child Neurol 2011;26:1203-6. [Crossref] [PubMed]
- Jordan LC, Hillis AE. Challenges in the diagnosis and treatment of pediatric stroke. Nat Rev Neurol 2011;7:199-208. [Crossref] [PubMed]
- Elbers J, Armstrong D, Benseler SM, et al. The Utility of Collaterals as a Biomarker in Pediatric Unilateral Intracranial Arteriopathy. Pediatr Neurol 2018;78:27-34. [Crossref] [PubMed]
- Stence NV, Pabst LL, Hollatz AL, et al. Predicting Progression of Intracranial Arteriopathies in Childhood Stroke With Vessel Wall Imaging. Stroke 2017;48:2274-7. [Crossref] [PubMed]
- Dlamini N, Yau I, Muthusami P, et al. Arterial Wall Imaging in Pediatric Stroke. Stroke 2018;49:891-8. [Crossref] [PubMed]
- Perez FA, Oesch G, Amlie-Lefond CM. MRI Vessel Wall Enhancement and Other Imaging Biomarkers in Pediatric Focal Cerebral Arteriopathy-Inflammatory Subtype. Stroke 2020;51:853-9. [Crossref] [PubMed]


