
Exogenous endothelial progenitor cells reached the deficient region of acute cerebral ischemia rats to improve functional recovery via Bcl-2
Introduction
Acute cerebral ischemic stroke (AIS) results from the thrombotic occlusion of a major cerebral artery and the subsequent reduction in blood flow, which causes local cell hypoxia and necrosis (1). AIS patients experience long-term stroke-related sequelae, including paralysis, aphasia, dementia, post-stroke depression, and diminished quality of life (1,2). The foremost treatment for AIS involves restoring the cerebral blood flow and preventing further cerebral injury (1). While no current therapies can rapidly restore the microcirculation after ischemia (1,3), angiogenesis has been found to increase the cerebral blood flow and promote better prognosis by restoring cerebral oxygen and nutrition supply and participating in neuron protection and remodeling (3,4).
Endothelial progenitor cell (EPC) therapy holds promise in promoting angiogenesis and re-endothelialization for vascular lesions (5,6). Increasing the EPC transplant dose may be one solution for improving neuroprotective outcome, but excessive exogenous cells may hinder the outcome (7,8). EPC enhancement may address these shortcomings. Our previous research showed that autologous EPCs could protect acute focal ischemia rat via promoting angiogenesis (5,6). However, whether those EPCs that reached the deficient region were the transplanted ones or the products of other autoconversion they had promoted remains unknown (5,7). Presently, no research exists that offers direct evidence for the supposition that exogenous transplanted EPCs directly participate in angiogenesis in ischemic areas. However, our previous studies did find that EPCs can alleviate the apoptosis of nerve cells in ischemic brain tissue (5,6). As we know that B-cell lymphoma 2 (Bcl-2) is a classical apoptosis factor of nerve cells, we further detected the expression of this Bcl-2 pathway.
In this study, first we selected male Sprague-Dawley (SD) rats as a source for culturing the EPCs, and introduced them to middle cerebral artery occlusion (MCAO) female rats subsequently. We then observed the EPC activity in protecting the ischemic brain. More importantly, we traced the exogenous EPC process by using sex-determining region Y (SRY) gene in-situ hybridization (ISH). Lastly, we examined the expression of Bcl-2 to potentially reveal the possible mechanism. In this way, our study, for the first time, aimed at providing direct evidence for exogenous transplanted EPCs directly participating in angiogenesis in ischemic areas via the Bcl-2 pathway. We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/cdt-20-329).
Methods
EPC extraction, transfection, and culture
Mononuclear cells (MNCs) were isolated using a Ficoll gradient (Lympholite-M, Cedarlane) (9,10). In brief, MNCs were isolated by density-gradient centrifugation and resuspended in 10 mL of full endothelial cell culture medium. Cell suspensions were divided into two 5-cm2 culture flasks at 37 °C and 5% CO2. After 48 h of incubation, the non-adherent cells were eliminated. The fresh culture medium in the flasks was replaced every 3 days. Seven days after extraction, half of the cells were taken for transfection. Cell density was adjusted to 106/mL, and the cells were planted 1 mL per well in a 6-well plate.
MCAO and sham operation procedure
The rats in the MCAO group underwent an operation based on the previous studies (5,11). The left side of the middle cerebral artery was blocked with a surgical nylon monofilament. The rats in the sham operation group were anesthetized by intraperitoneal injection of 10% chloral hydrate and underwent the same surgical protocol except for the nylon thread input. The rats in the sham and MCAO-placebo groups received 0.5 mL of phosphate buffered saline (PBS) for 1 h after the operation. The rats in the MCAO-EPC group received 0.5 mL of EPC suspension. The EPCs were injected via the caudal vein.
Fifty SD rats, aged 1 or 2 months old, were purchased from STA Experimental Animal Company (Hunan, China). Of these, 10 one-month-old male SD rats were used for bone-marrow extraction, as younger animals have a higher EPC concentration in bone marrow. When the other 32 two-month-old female rats were of sufficient body weight (240–280 g), they were divided randomly into the sham operation group (n=8), the MCAO-placebo group (n=8), and the MCAO-EPC group (n=8). A total of 54 spontaneously hypertensive rats were used in this study. Forty-five rats received MCAO model surgery, 15 rats received sham surgery, and 11 died or were excluded from the experiment.
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures involving animals were performed according to an institutionally approved protocol in accordance with Laboratory Animal regulation of China (2015). The experiments were approved by the Medical Ethics Committee of Zhongnan Hospital of Wuhan University.
Cell transplantation
After EPC culture, the cells were cultured for about a week and then harvested in centrifuge tubes. Rats from the EPC treatment group were injected with 2×106 cells in 0.5 mL PBS via the tail vein after 2 h of MCAO reperfusion, consecutively for 7 days, while the control group was only treated with 0.5 mL PBS.
Behavioral test
A rotarod (TOR) test (12,13) was performed 6 days before the operation to establish a baseline of motor coordination. All rats were trained for 5 days before the operation, and the day before the operation was defined as day 0. Rats were placed on a rotarod cylinder accelerating from 4 to 40 rpm in 60 seconds, and the time that the rats remained on the cylinder was measured. Each rat was trained 3 times daily for 5 days. On day 6, the TOR times of all the rats were measured 3 times and the rats with low TOR time (<20 s) were excluded. The same test was performed on postoperative days 1, 3, 7, and 14 to record the TOR of each group.
Brain magnetic resonance imaging (MRI) to assess infarct volume and the gliosis status
Brain MRI (7.0 T) was performed on postoperative day 15 to determine the ischemic zone and the gliosis status. Rats from each group were randomly selected. Before MRI, the rats were anesthetized with 10% chloral hydrate and restrained in the MRI machine. The MRI protocol consisted of 13 T2-weighted images, which allowed the ischemic lesion volumes and the gliosis status to be evaluated (14,15).
Immunofluorescence and ISH to trace the transplanted EPCs
Immunofluorescence and ISH was performed according to the manufacturer’s instructions. CD31 antibody was used to determine the presence of the transplanted male SD rat-derived EPCs (16) in the ischemic zone, which allowed for a comparison of the microcirculation and angiogenesis (17) between groups.
Because we transplanted male EPCs to female rats, the SRY gene could be used as a probe to distinguish exogenous cells (18). The ISH test indicated that the majority of exogenous EPCs had settled in the ischemic lesion. Each section was imaged in cortical ischemic zone 5, and CD31-positive cells were counted as mean ± SD (standard deviation).
Immunohistochemistry
Immunohistochemistry was used to assess Bcl-2 expression in the ischemic brain, and was performed according to the manufacturer’s instructions, as we reported previously (5,11). Each section was imaged in cortical ischemic zone 5, and Bcl-2-positive cells were counted as mean ± SD.
Western blot detected the expression of Bcl-2 in ischemic brain tissue
Western blot was performed according to standard protocols (19,20) to test the level of the Bcl-2 proteins (21). Lysates were incubated on ice for 10 min and then centrifuged at 12,000 ×g for 5 min at 4 °C. An equal volume of 2× SDS-PAGE sample loading buffer was added to the lysates and heated at 100 °C for 5 min. Then, 40 µg of total protein from each sample was separated by SDS-PAGE gel, transferred to a nitrocellulose membrane, and detected with Bcl-2 antibodies. The gray value of every specimen was analyzed by SPSS 19.0 statistical software to determine if there was a significant difference (P<0.05).
Statistical analyses
All results were expressed as mean ± SD. Differences among groups were assessed by analysis of variance with Scheffe’s post-hoc test. A difference of P<0.05 was considered to be significant, and regression analysis used the relevant factors. All statistical calculations were completed using the SPSS19.0 software, with P<0.01 indicating a statistically significant difference, P<0.05 indicating the presence of statistical difference, and P>0.05 indicating no statistical difference.
Results
EPC characterization
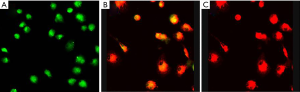
EPC, as precursors to endothelial cells, have the typical endothelial cell functions of uptaking acetylated low-density lipoprotein (ac-LDL) and Ulex europaeus agglutinin 1 (UEA-1). After 7 days of culture, we observed that the cells were double positive for Dil-ac-LDL uptake and FITC1-UEA-1 binding (Figure 1).
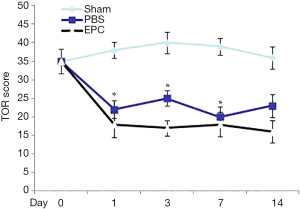
Cellular therapy promoted motor coordination on postoperative days 7 and 14
Safety observation showed no obvious adverse reaction was seen in the two groups of rats. The TOR baseline test on day 0 showed no significant difference among the groups. On days 1 and 3, the two groups showed a significant decrease of TOR compared to day 0 (P<0.05) and showed worse performance compared to the sham group (P<0.05). On days 3, 7, and 14, however, the EPC group showed superior motor coordination compared to the PBS group (P<0.05) (Figure 2).
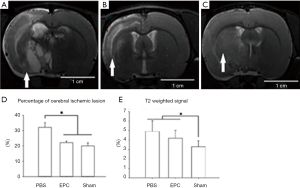
Cellular therapy reduced ischemic volume and gliosis status during the acute stage
Brain MRI findings evaluated ischemic lesion volume and gliosis status on postoperative day 14 (Figure 3A,B,C). Ischemic volume was measured and expressed as the percentage of all cerebral volume. Analysis showed that the ischemic volume of the MCAO-placebo group was 32.1%±3.0% of the whole brain, while that of the EPC group was 22.1%±1.3%. The PBS group had severe ischemic lesions and gliosis (Figure 3A), while the EPCs group showed less significant ischemic lesions (Figure 3B). The ischemic volume of the two cellular therapy groups showed significant difference (*P<0.05) (Figure 3D). The gliosis status could be evaluated by the T2-weighted signal of the ischemic cortex. The EPC group (3.332±0.918) showed a significantly less severe gliosis status (P<0.05) than the PBS group (4.896±1.143) (Figure 3E).
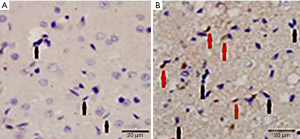
Detection of ectogenic cells in the female operation rat ischemic brain issue
ISH was performed to detect the ectogenic cells located in the same area (Figure 4), and the SRY gene-positive cell number of the EPCs group was (39.8±18.8), while the PBS-sham-group results were negative.
Transplanted-EPCs induced angiogenesis in ischemic brain issue
CD31 antibody was used to detect the newly developed endothelial cells and to evaluate capillary density within the ischemic zone (Figure 5A,B,C). When observed under 200× magnification, the PBS group (58.8±26.9) showed the fewest positive cells, and the EPC group (285.7±38.4) showed superior angiogenesis outcome compared to the PBS group (P<0.05) (Figure 5D).
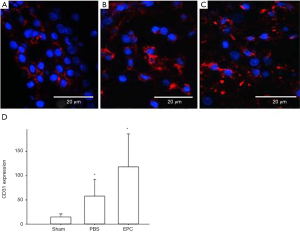
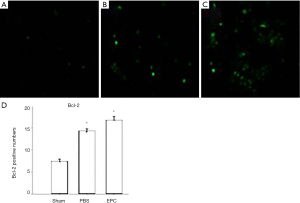
Effect of EPCs on the expression of Bcl-2 in the infarcted hemisphere
Ischemic tissue was collected from the animals 14 days after surgery.
First, immunohistochemistry was used to assess the expression of Bcl-2 in the ischemic brain. The figures are representative of at least four independent experiments with similar results. Immunohistochemical staining for Bcl-2 in the ischemic area showed an increased number of Bcl-2-positive cells in EPC-treated animals. The number of Bcl-2-positive cells was also found to be significantly increased in the EPC group than that in the sham groups (Figure 6).
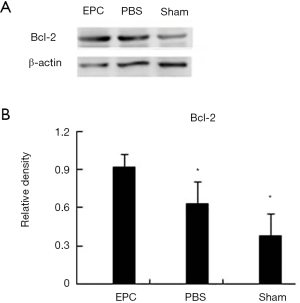
Western blot analysis showed increased levels of Bcl-2 in the brain tissue of EPC-treated animals (P<0.01) (Figure 7A). This result confirms that EPC transplantation could induce the expression of Bcl-2 in ischemic brain tissue (Figure 7B).
Discussion
In this study, we sourced EPCs exogenously from male rats. Meanwhile, we isolated mononuclear bone cells and differentiated them into EPCs using defined growth factors in vitro. EPCs were identified by cell surface markers (CD34+, CD45, and CD133+) and the uptake of ac-LDL and UEA-I (22,23). Using an acute focal cerebral ischemic model, we demonstrated that EPCs can increase endothelial cell proliferation and enhance recovery of neurological function after ischemic stroke, which is consistent with the results of our previous research (5,6). In these past studies, we demonstrated that cellular therapy is beneficial for acute cerebral ischemic injury during the acute stage (5,6).
In order to provide direct evidence for exogenously transplanted EPCs directly participating in angiogenesis in ischemic areas, immunofluorescence of CD31 and SRY gene ISH tests were conducted to trace the transplanted EPC process in the female animals. It is known that vascular damage and impaired collateral circulation contribute to ongoing brain injury and poor prognosis, while angiogenesis may enhance local microvascular circulation (24) by improving oxygen and nutrition provisioning to the para-ischemic zone. CD31 was found to be a specific molecular marker in endothelial cells (25), and CD31 antibody has been used to determine the presence of EPCs in the ischemic zone (16). Our results indicate that there were more CD31-positive cells in the EPC therapy groups than in the PBS group (P<0.05). Since female animals do not have a Y chromosome, the detected Y chromosome should be exogenous. The SRY gene ISH test detected the exogenous cells from the male rats (P<0.05), which indicates that the EPCs can relocate to the ischemic lesion rather than to the peripheral region directly. It remains unknown whether this advantage in concentration is due to its superiority in proliferation or migration ability (26,27). Two possible mechanisms may be considered: the transplanted EPCs could have migrated to the ischemic area, or the bone marrow EPCs could have migrated to the ischemic border area. Our results provide direct proof that the transplanted EPCs could migrate to the ischemic area. In addition, we found that the transplantation of EPC results in higher Bcl-2 levels, suggesting that Bcl-2 has an important role in the migration or recovery process.
Bcl-2 is a classical apoptosis factor of nerve cells and may also be an anti-inflammatory factor, so we further detected the expression of the Bcl-2 pathway. According to Song et al., the expression of Bcl-2 may increase and result in better outcome after MCAO injury (28). Also, Chen et al. demonstrated that EPC therapy could also promote the increase of Bcl-2 (29). In our study, the cellular therapy groups displayed an induced overexpression of Bcl-2, and a high EPC level further enhanced this effect. This suggests that the EPCs may have a stronger anti-apoptosis role and could thus possibly provide a better prognosis, which is consistent with our previous research results (5,6).
Some limitations to our study should also be addressed. These include a lack of long-term evaluation for the safety of the selected allografts in rats. There was also a limited amount of EPCs selected in this study that directly entered the ischemic brain tissue of rats. Furthermore, the method of culturing the EPCs might have had an effect on subsequent experiments, while an in vitro method to enhance the EPC activity in rats is still needed for further intervention. All of these issues are currently being considered and may be addressed in future research.
Conclusions
EPCs promote angiogenesis and the restoration of blood supply, which can reduce the scope of ischemia and prevent neuronal infarction (30). Compared with other therapeutic strategies such as the use vascular endothelial growth factor (VEGF) (31,32) and granulocyte-colony stimulating factor (G-CSF) (33-35), EPC transplantation holds more advantages. EPCs are easy to process, can be obtained from a wide variety of sources, and can circumvent immune rejection. Our results suggest the potential clinical applications for EPC transplantation in ischemic stroke, which may provide a simple, quick, and safe alternative to current cell-based therapies. Furthermore, we have gathered evidence which supports the effectiveness of EPCs and suggests this cellular therapy could be beneficial to ischemic stroke patients in the future.
Acknowledgments
Funding: This study was supported by grants from the National Natural Science Foundation of China given to Dr. Yumin Liu (No. 81371273).
Footnote
Reporting Checklist: The authors present the study in accordance in accordance with the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/cdt-20-329
Data Sharing Statement: Available at http://dx.doi.org/10.21037/cdt-20-329
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-329). All authors report grants from the National Natural Science Foundation of China, outside the submitted work.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures involving animals in this study were performed according to an institutionally approved protocol in accordance with Laboratory Animal regulation of China (in 2015). The experiments were approved by the Medical Ethics Committee of Zhongnan Hospital of Wuhan University.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hong KS, Ko SB, Lee JS, et al. Endovascular Recanalization Therapy in Acute Ischemic Stroke: Updated Meta-analysis of Randomized Controlled Trials. J Stroke 2015;17:268-81. [Crossref] [PubMed]
- Kong Z, Jiang J, Deng M, et al. Edaravone reduces depression severity in patients with symptomatic intracranial stenosis and is associated with the serum expression of sex hormones. Medicine (Baltimore) 2020;99:e19316. [Crossref] [PubMed]
- Gunsilius E, Petzer AL, Stockhammer G, et al. Serial measurement of vascular endothelial growth factor and transforming growth factor-beta1 in serum of patients with acute ischemic stroke. Stroke 2001;32:275-8. [Crossref] [PubMed]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med 2000;6:389-95. [Crossref] [PubMed]
- Kong Z, Hong Y, Zhu J, et al. Endothelial progenitor cells improve functional recovery in focal cerebral ischemia of rat by promoting angiogenesis via VEGF. J Clin Neurosci 2018;55:116-21. [Crossref] [PubMed]
- Zhang R, Xie X, Yu Q, et al. Constitutive Expression of Adiponectin in Endothelial Progenitor Cells Protects a Rat Model of Cerebral Ischemia. Neural Plast 2017;2017:6809745.
- Li DW, Liu ZQ, Wei J, et al. Contribution of endothelial progenitor cells to neovascularization Int J Mol Med 2012;30:1000-6. (Review). [Crossref] [PubMed]
- Wang LQ, Lin ZZ, Zhang HX, et al. Timing and dose regimens of marrow mesenchymal stem cell transplantation affect the outcomes and neuroinflammatory response after ischemic stroke. CNS Neurosci Ther 2014;20:317-26. [Crossref] [PubMed]
- Kamiya N, Ueda M, Igarashi H, et al. Intra-arterial transplantation of bone marrow mononuclear cells immediately after reperfusion decreases brain injury after focal ischemia in rats. Life Sci 2008;83:433-7. [Crossref] [PubMed]
- Hisatome T, Yasunagaa Y, Yanadaa S, et al. Neovascularization and bone regeneration by implantation of autologous bone marrow mononuclear cells. Biomaterials 2005;26:4550-6. [Crossref] [PubMed]
- Kong Z, Shen Q, Jiang J, et al. Wogonin improves functional neuroprotection for acute cerebral ischemia in rats by promoting angiogenesis via TGF-β1. Ann Transl Med 2019;7:639. [Crossref] [PubMed]
- Bohlen M, Cameron A, Metten P, et al. Calibration of rotational acceleration for the rotarod test of rodent motor coordination. J Neurosci Methods 2009;178:10-4. [Crossref] [PubMed]
- Minnerup J, Seeger FH, Kuhnert K, et al. Intracarotid administration of human bone marrow mononuclear cells in rat photothrombotic ischemia. Exp Transl Stroke Med 2010;2:3. [Crossref] [PubMed]
- Fitzgerald RT, Ou X, Nix JS, et al. Dodecafluoropentane emulsion delays and reduces MRI markers of infarction in a rat stroke model: a preliminary report. Magn Reson Imaging 2015;33:236-9. [Crossref] [PubMed]
- Jiang Q, Thiffault C, Kramer BC, et al. MRI detects brain reorganization after human umbilical tissue-derived cells (hUTC) treatment of stroke in rat. PLoS One 2012;7:e42845. [Crossref] [PubMed]
- Shim Y, Nam MH, Hyuk SW, et al. Concurrent hypermulticolor monitoring of CD31, CD34, CD45 and CD146 endothelial progenitor cell markers for acute myocardial infarction. Anal Chim Acta 2015;853:501-7. [Crossref] [PubMed]
- Clarkson AN, Liu H, Schiborra F, et al. Angiogenesis as a predictive marker of neurological outcome following hypoxia-ischemia. Brain Res 2007;1171:111-21. [Crossref] [PubMed]
- Hess DC, Hill WD, Martin-Studdard A, et al. Bone marrow as a source of endothelial cells and NeuN-expressing cells After stroke. Stroke 2002;33:1362-8. [Crossref] [PubMed]
- Rong JJ, Sang HF, Qian AM, et al. Biocompatibility of porcine small intestinal submucosa and rat endothelial progenitor cells in vitro. Int J Clin Exp Pathol 2015;8:1282-91. [PubMed]
- Shimamura N, Matchett G, Yatsushige H, et al. Inhibition of integrin alphavbeta3 ameliorates focal cerebral ischemic damage in the rat middle cerebral artery occlusion model. Stroke 2006;37:1902-9. [Crossref] [PubMed]
- Qi Z, Dong W, Shi W, et al. Bcl-2 phosphorylation triggers autophagy switch and reduces mitochondrial damage in limb remote ischemic conditioned rats after ischemic stroke. Transl Stroke Res 2015;6:198-206. [Crossref] [PubMed]
- Zhang P, Li J, Liu Y, et al. Human neural stem cell transplantation attenuates apoptosis and improves neurological functions after cerebral ischemia in rats. Acta Anaesthesiol Scand 2009;53:1184-91. [Crossref] [PubMed]
- Lin FY, Tsao NW, Shih CM, et al. The biphasic effects of oxidized-low density lipoprotein on the vasculogenic function of endothelial progenitor cells. PLoS One 2015;10:e0123971. [Crossref] [PubMed]
- Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell 2011;146:873-87. [Crossref] [PubMed]
- Lee S, Valmikinathan CM, Byun J, et al. Enhanced therapeutic neovascularization by CD31-expressing cells and embryonic stem cell-derived endothelial cells engineered with chitosan hydrogel containing VEGF-releasing microtubes. Biomaterials 2015;63:158-67. [Crossref] [PubMed]
- Nakamura N, Naruse K, Matsuki T, et al. Adiponectin promotes migration activities of endothelial progenitor cells via Cdc42/Rac1. FEBS Lett 2009;583:2457-63. [Crossref] [PubMed]
- Shibata R, Skurk C, Ouchi N, et al. Adiponectin promotes endothelial progenitor cell number and function. FEBS Lett 2008;582:1607-12. [Crossref] [PubMed]
- Song W, Huo T, Guo F, et al. Globular adiponectin elicits neuroprotection by inhibiting NADPH oxidase-mediated oxidative damage in ischemic stroke. Neuroscience 2013;248:136-44. [Crossref] [PubMed]
- Chen YL, Tsai TH, Wallace CG, et al. Intra-carotid arterial administration of autologous peripheral blood-derived endothelial progenitor cells improves acute ischemic stroke neurological outcomes in rats. Int J Cardiol 2015;201:668-83. [Crossref] [PubMed]
- Nagasawa H, Yokota C, Toyoda K, et al. High level of plasma adiponectin in acute stroke patients is associated with stroke mortality. J Neurol Sci 2011;304:102-6. [Crossref] [PubMed]
- Zhang ZG, Zhang L, Jiang Q, et al. VEGF enhance angiogesis and promote blood-brain barrier leakage in the ischemic brain. J Clin Invest 2000;106:829-38. [Crossref] [PubMed]
- Schmidt NO, Koedera D, Messinga M, et al. Vascular endothelial growth factor-stimulated cerebral microvascular endothelial cells mediate the recruitment of neural stem cells to the neurovascular niche. Brain Res 2009;1268:24-37. [Crossref] [PubMed]
- Lee ST, Chua K, Jung KH, et al. Granulocyte colony-stimulating factor enhances angiogenesis after focal cerebral ischemia. Brain Res 2005;1058:120-8. [Crossref] [PubMed]
- Yan H, Changsheng D, Junjian Z, et al. Neuroprotective effective of Granulocyte Colony-stimulating Factor in a focal cerebral ischemic rat model with hyperlipidemia. J Huazhong Uni Sci Technol 2012;32:872-8. [Crossref]
- Schäbitz WR, Kollmar R, Schwaninger M, et al. Neuroprotective effect of granulocyte colony-stimulating factor after focal cerebral ischemia. Stroke 2003;34:745-51. [Crossref] [PubMed]


