
Early clinical outcomes of thoracoscopic mitral valvuloplasty: a clinical experience of 100 consecutive cases
Introduction
Minimally invasive cardiac surgery is the trend of modern medicine, which has developed rapidly in the past decade. In 1996, Carpentier et al. (1) completed the first case of mitral valvuloplasty with success under video surgery and minithoracotomy, which opened the prologue of thoracoscopic valvular surgery. In the past 20 years, minimally invasive cardiac procedures including lower hemisternotomy, direct-vision right minithoracotomy and thoracoscopic assisted right minithoracotomy are the standard approach in many centers in regards of their superiority of reduced trauma, decreased bleeding, hastened recovery and improved cosmetics with high patient satisfaction compared to conventional MV surgery through a median sternotomy (2). Adoption of an “endoscopic” approach to port access mitral valvuloplasty provides a “direct view” of the valve with excellent visualization that allows a systematic, complete assessment of mitral leaflet morphology as well as the subvalvular apparatus. Once it was applied in clinical, it has been widely concerned, gradually promoted, and rapidly developed. However, up till now, relatively few centers can carry out totally thoracoscopic mitral valvuloplasty independently (3,4). We summarized the clinical experience of 100 consecutive cases of mitral valvuloplasty in the early period, and evaluated its safety and efficacy by its early clinical outcomes. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/cdt-20-440).
Methods
The research was conducted in accordance with the Declaration of Helsinki (as was revised in 2013). This study is a normal clinical practice without any special intervention for patients, all patients had previously granted permission for use of their medical records for research purposes, and our institutional committee on human research approved the study protocol. Unfortunately, we do not have the ID, which maybe a shortage of the article.
Patients
Between September 2017 and December 2019, 100 consecutive cases of thoracoscopic mitral valvuloplasty (including 74 cases of totally thoracoscopy) had been completed by one experienced surgeon in the heart center of Chinese PLA General Hospital, accounting for 35.3% of thoracoscopic cardiac surgery in the same period. There were 56 males and 44 females with an average age of 49.2±14.7 (15 to 75) years. The lesions consisted of 78 cases of degenerative valvular disease (including 10 cases of Barlow's disease), 10 congenital valvular cleft, 9 infective endocarditis, 2 rheumatic valvular disease and 1 congenital papillary muscle abnormality. Clinical characteristics of the patients were present in Table 1. Preoperative coronary angiography reveled coronary heart disease in 13 cases, all of which had no indication of interventional therapy or surgical treatment.
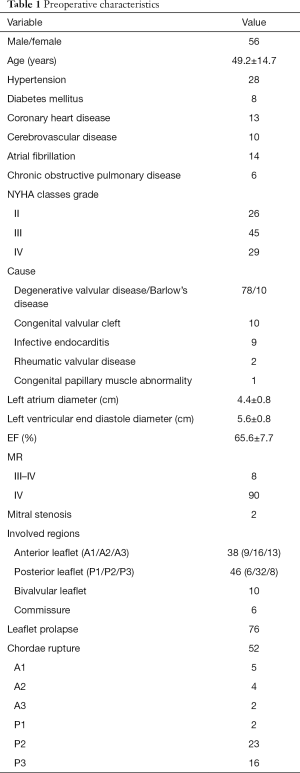
Full table
Surgical technique
After the induction of general anaesthesia, a left-sided double-lumen endotracheal tube was placed to allow for single-lung ventilation. Placement of defibrillator pads across the chest wall was routine, as access to the ventricles was limited. The patients were positioned supine all the way to the right side of the table with the right hemithorax elevated to 30° and right arm tucked at the side to improve access to the anterior axillary line. A small pillow was placed inferior to the scapula to open up the axillary space. A TEE probe was then placed to evaluate valvular and ventricular function before and after surgery in all patients.
The CPB was established through femoral artery, femoral vein, and right internal jugular vein. The main operating hole (about 3.5 cm), through which the cardioplegia irrigation tube was also passed, was located in the fourth intercostal space outside the right midclavicular line. The thoracoscopy was placed through the third intercostal space on the right anterior axillary line, while the left cardiac drainage and Chitwood clamp were punctured through the fourth intercostal space on the right midaxillary line. The left atrium was raised by a blade retractor and introduced through a 2 mm shaft penetrating parasternally in the fourth intercostal space and was held on the operating table. The MV was exposed by means of traditional left atrium incision, which was parallelly to the interatrial sulcus, otherwise right atrial incision was used in patients with atrial septal defect repair or tricuspid valve surgery. CO2 insufflation was used routinely.
Follow-up
All patients were examined by TTE to evaluate the recovery of cardiac function one week after operation. All patients discharged were followed up by postoperative examination back to hospital every 0.5–1 year after operation and by telephone call or a written questionnaire. Data obtained included TTE, functional status, survival, and cardiac related hospital re-admission. Details of surgery and postoperative data were present in Table 2.
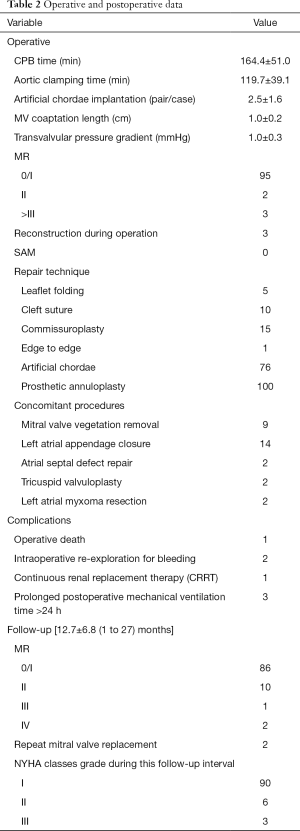
Full table
Statistical analysis
The data were analyzed by SPSS 23.0 Software (SPSS, Inc., Chicago, IL, USA). Continuous variables were expressed as mean and standard deviation. Categorical variables were presented as percentages. The Student t test and variance analysis were used to compare continuous variables, and the chi-squared test and Fischer’s exact test were used for analysis of categorical variables. A significant difference was considered at P<0.05.
Results
Operation and postoperative data
MV repair was performed by means of Carpentier techniques, including leaflet folding in 5 cases, cleft suture in 10, commissuroplasty in 15 including 2 commissurotomy, edge to edge in 1, and artificial chordae implantation in 76 cases with an average of 2.5±1.6 (1 to 4) pairs. Prosthetic annuloplasty ring was used in all cases, including 36 Physio II and 64 Cosgrove, with an average size of 31.4±2.1 (28 to 36) mm and 31.7±1.6 (28 to 34) mm, respectively. Intraoperative TEE revealed no MR in 95 cases and a mild in 2 cases with all coaptation length more than 5 mm. The rest 3 cases with moderate or more MR were successfully reconstructed during a second pump-run. Concomitant procedures included MV vegetation removal in 9 cases, left atrial appendage closure in 14, and 2 cases of atrial septal defect repair, tricuspid valvuloplasty and left atrial myxoma resection, respectively. The average CPB time was 164.4±51.0 min and aortic clamping time was 119.7±39.1 min, and the latest 10 cases were 140.2±45.3 min and 96.3±25.4 min, respectively (P<0.05), with similar changes in postoperative chest drainage in the first 24h (232.8±108.1 vs. 140.2±45.3 ml, P<0.05), blood products use (2.2±1.8 vs. 0.9±0.6 U, P<0.05), mechanical ventilation length (13.2±6.2 vs. 9.9±6.4 h, P<0.05), ICU stay (2.9±2.2 vs. 1.3±1.1 d, P<0.05), and postoperative hospital stay (6.2±6.4 vs. 4.7±2.1 d, P<0.05), as presented in Table 3.

Full table
There was only one operative death from avulsion of left atrial suture 4 hours after operation. Intraoperative re-exploration through a conversion to sternotomy for bleeding was performed in 2 cases, including an innominate artery injury caused by superior vena cava cannulation, and a left atrial appendage injury due to Chitwood clamp. One case received continuous renal replacement therapy (CRRT) for postoperative acute renal failure, and 3 cases suffered prolonged postoperative mechanical ventilation time more than 24 hours.
Follow-up
All patients discharged were successfully followed up until December 2019 with a mean follow-up of 12.7±6.8 (1 to 27) months. Functional classification improved, as NYHA status changed from the preoperative mean of 3.03 to 1.12 during this follow-up interval. Severe MR was observed in 2 patients 3 months after operation, and MVR was performed through median sternotomy. Up to the recent follow-up, there were 86 cases with no or trace MR, 10 with a mild, and 1 with a moderate.
Discussion
In the past 20 years, modern surgery has made remarkable progress. In particular, with the advantage of damage control surgery, minimally invasive surgery, fast-track surgery and extracorporeal life support, the essence of modern surgery has shifted from the traditionally-held “operation treating disease” to modern “minimizing the damage” (5). MV repair has become the preferred procedure of choice for the treatment of MR, with superior results relative to MVR in terms of very low operative mortality, improved quality of life and excellent long-term survival (6). The application of minimally invasive technology in cardiac surgery has greatly changed the surgical approaches of MV repair. Even with complex MR, totally thoracoscopic MV repair can achieve satisfied surgical effect, which could be completely comparable to those by median sternotomy (7). However, the totally thoracoscopic MV repair is still technically challenging, and its application is currently restricted to a handful of experienced operators because it entails the surgeon overcoming a lengthy learning curve (8).
Accumulating knowledge of the structure, function, and pathology of the MV, in combination with a better appreciation of risks associated with untreated severe MR and valve replacement, has dramatically changed the indications of surgery during the last decade (9). An early intervention in asymptomatic severe MR is indicated only if the surgeon is assured of the feasibility of a reconstructive operation (9), with their survival found to be identical to that of general population after operation (10). The ultimate aim of valve reconstruction is to restore durable normal valve function guided by functional valve analysis. Three basic Carpentier’s principles are required to achieve this goal: (I) preserving or restoring normal leaflet motion of all valve segments, (II) creating a large surface of leaflet coaptation, (III) remodeling the annulus to provide an optimal and stable orifice area (9).
MV repair is feasible in as many as 95% of cases of degenerative MR, despite the presence of complex lesions (11). The Carpentier quadrangular resection, with or without concomitant sliding plasty, is considered the standard surgical technique to correct posterior leaflet prolapse (12). Various types of reconstructive procedures, including leaflet triangular resection, chordae transposition/replacement/shortening, and papillary muscle sliding/shortening, have been used to repair anterior leaflet prolapse (12). However, MV repairs with these techniques are not always satisfactory, and some of these procedures are considered technically demanding. Since the introduction of standardized techniques for MV reconstruction by Carpentier and others, numerous technical improvements have been made in MV repair in the last 20 years, particularly in the treatment of anterior and bileaflet MV prolapse. Efforts to find a substitute for elongated or ruptured chordae date back to the 1960s. In 1962, January et al. (13) described a patient operated on by placing two 00 sutures through the base of papillary muscles and through the margins of the unsupported portion of the mural leaflet. Frater et al. (14,15) in 1965 published their experience with experimental and clinical use of autologous pericardium as a chordal substitute, and introduced chordae replacement with Gore-Tex sutures experimentally in the early 1980s. Literature studies have confirmed the long-term good results of the artificial chordae in MV repair and considered it as an effective method widely used in clinical to solve the complex MV diseases such as valve prolapse and Barlow’s disease for its avoidance of leaflet resection and increase of leaflet coaptation (6,16). Due to the difficulty of leaflet resection and suture under thoracoscopy, we usually use relatively simple techniques, such as leaflet plication, commissural suture, and multiple artificial chordae implantation to correct the leaflet prolapse and create a large surface of coaptation. In our series, degenerative MV disease was the most common cause, which comprised 78%. Artificial chordae were the most common used technique, accounting for 76% with an average number of 2.5±1.6 (1 to 4) pairs, whereas 100% used in Barlow’s patients with an average number of 3.4±0.7 (2 to 4) pairs. The average MV leaflet coaptation was 1.0±0.2 cm without any SAM, which fully showed the advantages of artificial chordae technique.
The remodeling annuloplasty ring, which can not only restore the normal systolic shape and size of the annulus to a needed condition for the optimal coaptation, but also prevent further deformation for the preference to ensure the long-term stability of the annulus, is considered to be both an important auxiliary means of valvular repair and an important guarantee for long-term good results (9). In our series, prosthetic annuloplasty ring was used in all cases, including 36 Physio II and 64 Cosgrove, with an average size of 31.4±2.1 (28 to 36) mm and 31.7±1.6 (28 to 34) mm, respectively. Annular contraction, which is a consequence of ventricular contraction, is to facilitate the competency of the MV by reducing the orifice area by 20–30%, which in turn increases the surface area of leaflet coaptation (9). Based on this design concept, the physio II annuloplasty ring was given a systolic configuration (9). However, this rigid complete ring was designed at the expense of annular diastolic function despite with a semirigid and shock-absorbing structure to compensate. Nevertheless, in certain cases of MV repair, due to a large valvular resection, a simple annular plication or compression suture and a sliding leaflet plasty was preferred in the corresponding posterior annulus in order to reduce the tension of the adjacent segments. The use of physio II annuloplasty ring could effectively prevent the leaflets at the adjacent segments from avulsion in the long-term. Surgical anatomy implies the anterior leaflet is primarily related to the left ventricular outflow tract via the aotto-mitral curtain whereas the posterior leaflet is related to the muscular parietal base of the left ventricle. As a result of this configuration, the maximum stress during systole is concentrated at the midline of the posterior leaflet (9). Therefore, some experts considered that only using a Cosgrove annuloplasty band to remodel the posterior annulus could also achieve an optimal and stable MV orifice with satisfied long-term results. At present, despite many reports on MV repair techniques, there is still a lack of consensus on whether to use a rigid ring or a flexible one, a complete or an incomplete one (17). Evidence based medical proofs in this regard is of paramount importance, calling for further multicenter, large-scale prospective randomized controlled studies.
We also successfully treated 10 cases of severe MR in Barlow's disease regardless of multisegmented involvement or limited leaflets prolapse. MV repair for this myxomatous disease involved leaflets, annulus, chordae, and papillary muscle motion in its complex form of pathology often requiring complex surgery, which was a particularly challenging procedure with less than optimal results (18,19). The surgery was begun with multiple artificial chordae implantation in P1, P2, and A2, or even A1 area routinely to decrease the height of leaflet and create enough leaflet coaptation as well as a normal ratio of A2/P2 height. Then we advanced the posterior commissure medially by two or three sutures to diminish excessive leaflet tissue and/or correct commissural prolapse. And finally, a 34 mm Cosgrove annuloplasty ring was placed. Thus, the three pathologic components contributing to MR; namely the leaflets, annulus, and chordae, were all surgically corrected. The mean CPB time was 142±26 min and aortic clamping time was 96±18 min. The average number of artificial chordae implantation was 3.4±0.7 (2 to 4) pairs. Intraoperative TEE confirmed successful repair in all patients with a mean MV coaptation length and transvalvular pressure gradient of 1.2±0.2 (0.8 to 1.5) cm and 1.2±0.4 (0.7 to 2.0) mmHg, respectively, and without any MR or SAM. During a follow-up of 1 to 18 months, TTE revealed 7 cases with no MR and 3 with trace MR, with a mean transvalvular pressure gradient of 1.5±0.6 (1.0 to 2.2) mmHg.
Previous studies have shown there is a significant learning curve of minimally invasive totally thoracoscopic cardiac surgery (8). We retrospectively analyzed the results of 100 consecutive cases of thoracoscopic MV repair in the early period, which had begun from thoracoscopic assist with a surgical incision of 6–9 cm, to totally thoracoscopy by the aid of soft tissue retractor with an incision of 4–6 cm, to finally with a 3.5 cm surgical incision. Further data study showed the mean time of CPB and aortic clamping in the first 10 cases was up to 388.8±105.6 and 284.4±80.5 min, respectively, while in the first 10 totally thoracoscopic cases, they decreased to 242.5±75.6 and 187.0±55.2 min, respectively, and in the latest 10 cases, they were 140.2±45.3 and 96.3±25.4 min, respectively, which were consistent with those of Guangdong Cardiovascular Institute and the heart center of Hamburg University (20,21), revealing a learning curve in totally thoracoscopic mitral valvuloplasty. Similar changes were observed in postoperative chest drainage in the first 24 h, blood products use, mechanical ventilation length, ICU stay, and postoperative hospital stay, which also indicated a learning curve in totally thoracoscopic mitral valvuloplasty.
According to the early clinical outcomes of the 100 consecutive cases of thoracoscopic mitral valvuloplasty, the surgeon had the following experience. (I) There was a learning curve in thoracoscopic mitral valvuloplasty. As we had observed previously, the indexes of evaluating MV repair learning process from quantitative to qualitative changes. At present, totally thoracoscopic mitral valvuloplasty could achieve satisfied results even in complex mitral diseases, such as Barlow’s disease. Secondly, thoracoscopic MV surgery needed continuous learning and experience accumulation. In our study, there were one case of operative death, and 2 cases of intraoperative re-exploration for bleeding and reoperation for recurrent MR, respectively. Based on constant running in and experience accumulation of the team, no serious complication occurred in the latter 50 cases of operation. (II) MV repair under thoracoscopy required simple and convenient techniques, as mentioned previously. Of course, this kind of simplicity was not simplification. MV repair must always follow the three fundamental principles of valve reconstruction, and must always aim to restore durable normal valve function. (III) A team effort made reconstructive valve surgery simple. Although the surgeon was at the center of this program, his efforts would be futile without the help and support of a team of specialists comprising cardiologists, echocardiographers, and anesthesiologists.
Conclusions
Totally thoracoscopic mitral valvuloplasty was technically feasible, safe, effective, and reproducible in clinical practice after crossing the learning curve. The short-term effect was satisfactory; however, further randomized, and long-term follow-up studies were warranted to determine its clinical effects.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors present the study in accordance with the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/cdt-20-440
Data Sharing Statement: Available at http://dx.doi.org/10.21037/cdt-20-440
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-440). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The research was conducted in accordance with the Declaration of Helsinki (as was revised in 2013). This study is a normal clinical practice without any special intervention for patients, all patients had previously granted permission for use of their medical records for research purposes, and our institutional committee on human research approved the study protocol.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Carpentier A, Loulmet D, Carpentier A, et al. Open heart operation under videosurgery and minithoracotomy. First case (mitral valvuloplasty) operated with success. C R Acad Sci III 1996;319:219-23. [PubMed]
- Sündermann SH, Czerny M, Falk V. Open vs. Minimally Invasive Mitral Valve Surgery: Surgical Technique, Indications and Results. Cardiovasc Eng Technol 2015;6:160-6. [Crossref] [PubMed]
- Chiu KM, Chen RJ. Videoscope-assisted cardiac surgery. J Thorac Dis 2014;6:22-30. [PubMed]
- Akowuah E, Burdett C, Khan K, et al. Early and Late Outcomes After Minimally Invasive Mitral Valve Repair Surgery. J Heart Valve Dis 2015;24:470-7. [PubMed]
- Li N. The enlightenment of the advances in modern surgery. Chin J Prac Surg 2015;35:1-3.
- Salvador L, Mirone S, Bianchini R, et al. A 20-year experience with mitral valve repair with artificial chordae in 608 patients. J Thorac Cardiovasc Surg 2008;135:1280-7. [Crossref] [PubMed]
- Bergsland J, Mujanovic E, Elle OJ, et al. Minimally invasive repair of the mitral valve: technological and clinical developments. Minim Invasive Ther Allied Technol 2011;20:72-7. [Crossref] [PubMed]
- Casselman FP, Van Slycke S, Wellens F, et al. Mitral valve surgery can now routinely be performed endoscopically. Circulation 2003;108 Suppl 1:II48-54. [Crossref] [PubMed]
- Carpentier A, Adams DH, Filsoufi F. Carpentier’s reconstructive valve surgery. Philadelphia: Saunders, 2010:48-133.
- David TE, Ivanov J, Armstrong S, et al. Late outcomes of mitral valve repair for floppy valves: Implications for asymptomatic patients. J Thorac Cardiovasc Surg 2003;125:1143-52. [Crossref] [PubMed]
- David TE, Ivanov J, Armstrong S, et al. A comparison of outcomes of mitral valve repair for degenerative disease with posterior, anterior, and bileaflet prolapse. J Thorac Cardiovasc Surg 2005;130:1242-9. [Crossref] [PubMed]
- Carpentier A. Cardiac valve surgery--the "French correction". J Thorac Cardiovasc Surg 1983;86:323-37. [Crossref] [PubMed]
- January LE, Fisher JM, Ehrenhaft JL. Mitral insufficiency resulting from rupture of normal chorade tendineae. Report of a surgically corrected case. Circulation 1962;26:1329-33. [Crossref] [PubMed]
- Frater RW, Berghuis J, Brown AL Jr, et al. The experimental and clinical use of autogenous pericardium for the replacement and extension of mitral and tricuspid value cusps and chordae. J Cardiovasc Surg (Torino) 1965;6:214-28. [PubMed]
- Zussa C (ed). Artificial chordae in mitral valve surgery (medical intelligence unit). Chapter 7: Artificial chordae Clinical Experience. Austin: RG Landes Co., 1994:79-112.
- Kobayashi J, Sasako Y, Bando K, et al. Ten-year experience of chordal replacement with expanded polytetrafluoroethylene in mitral valve repair. Circulation 2000;102:III30-4. [Crossref] [PubMed]
- Guenzinger R, Guenther T, Ratschiller T, et al. Is a Profiled Annuloplasty Ring Suitable for Repair of Degenerative Mitral Regurgitation? A Single-Center Experience Comprising 200 Patients. Thorac Cardiovasc Surg 2016;64:434-40. [PubMed]
- Lawrie GM. Barlow disease: Simple and complex. J Thorac Cardiovasc Surg 2015;150:1078-81. [Crossref] [PubMed]
- Ben Zekry S, Spiegelstein D, Sternik L, et al. Simple repair approach for mitral regurgitation in Barlow disease. J Thorac Cardiovasc Surg 2015;150:1071-7.e1. [Crossref] [PubMed]
- Zhu R, Huang H, Liu J, et al. Early outcomes of totally endoscopic mitral valve repair with artificial chordae implantation. Chin J Clin Thorac Cardiov Surg 2017;24:787-90.
- Westhofen S, Conradi L, Deuse T, et al. A matched pairs analysis of non-rib-spreading, fully endoscopic, mini-incision technique versus conventional mini-thoracotomy for mitral valve repair. Eur J Cardiothorac Surg 2016;50:1181-7. [Crossref] [PubMed]


