Warfarin anticoagulation in acute type A aortic dissection survivors (WATAS)
Introduction
Current surgical treatment of acute type A aortic dissection (AAAD) carries an in-hospital mortality between 17–37% (1). Those who undergo successful surgical repair of AAAD have a high mortality of 10% at 1 year, and of 23% to 28% at 5 years (2). Age, cerebrovascular accident, renal failure, previous cardiac surgery, extent of aortic replacement, and Marfan syndrome are well-established predictors of long-term survival after AAAD (2). In contrast to these well-investigated predictors, the role of warfarin anticoagulation on long-term outcome has not been elucidated.
It is reasonable to suggest an impact of warfarin anticoagulation on survival of AAAD. For example, in the acute state of AAAD, 98% of early fatalities result from hemorrhage through rupture of the aortic wall (3). Consequently, poor early survival of AAAD was documented with inadvertent thrombolytic therapy (4), and with aspirin or heparin (5) prior to emergent surgical repair.
Conversely, for the long-term outcome after surgical repair of AAAD the effect of warfarin anticoagulation was not documented. Consequently, some physicians suppose that warfarin increases the risk of aortic rupture (6), accelerates progression of dissection (7), and prevents aortic healing by hindering false lumen thrombosis (8). Other physicians presume that warfarin anticoagulation is likely to improve long-term survival of AAAD because it may prevent malperfusion or because it may avoid partial thrombosis of the false lumen (6,8). One study linked anticoagulation with improved long-term outcome of postsurgical AAAD. However, this study considered anticoagulation with all kinds of medication including aspirin, clopidogrel, and warfarin at non-therapeutic levels (8). This lack of evidence provides grounds for considerable variation of expert recommendations. Some experts consider warfarin anticoagulation as contraindication (6) and recommend to withhold anticoagulation in AAAD survivors despite atrial fibrillation (9), pulmonary embolism (10), or deep vein thrombosis (10). Other physicians consider warfarin anticoagulation mandatory to prevent organ malperfusion and therefore recommend warfarin as routine medication in all in AAAD survivors (6).
We performed a study of warfarin anticoagulation in AAAD survivors (WATAS) with the following objectives: first, to assess frequency and indications for long-term warfarin anticoagulation after surgical repair; second, to investigate whether warfarin anticoagulation was associated with increased long-term mortality or with increased late aortic events; third, to assess the relationship of warfarin anticoagulation with false lumen status or with annual growth of aortic diameters; finally, to assess classical predictors of long-term outcome in AAAD survivors. To these ends, we performed a long-term clinical follow-up in 243 survivors of AAAD at the surgical centers of Hamburg and Dresden.
Methods
Patients
We applied STROBE criteria for this retrospective, observational cohort study (11). The study recruited persons after emergent surgery for AAAD in two tertiary care centres of Hamburg and Dresden. All participants had spontaneous classical AAAD and they were recruited between September 2006 and November 2013. We only considered survivors with clinical follow-up after dismissal from our tertiary care centers. We classified all individuals according to postoperative initiation of life-long warfarin anticoagulation with a target INR >2. We excluded 134 persons due to absence of follow-up data, 7 because they were on new oral anticoagulants, and another 28 persons because of uncertain anticoagulation status during follow-up. Therefore, we studied a total of 155 men and 88 women at a mean age of 62±13 years (range 29–84 years). Of these, 136 persons were recruited in Hamburg and 107 in Dresden. The study was approved by regional ethics committee of Hamburg and Dresden, and informed consent was taken from all the patients.
Baseline characteristics
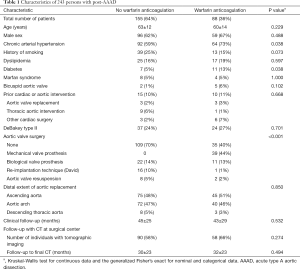
We assessed age at the time of operation for AAAD, chronic arterial hypertension, history of smoking, dyslipidemia, diabetes mellitus, and Marfan syndrome all as noted in the charts. A bicuspid aortic valve was diagnosed when two instead or three aortic valve cusps were described at surgery. We recorded prior cardiac or aortic intervention, and assessed extension of aortic dissection according to aortic segments as defined in current guidelines (12). We classified AAAD according to DeBakey as type I with ascending aortic dissection extending beyond, and as type II when restricted to the ascending aorta (12). We documented aortic valve intervention at AAAD repair with use of a mechanical valve, a bio-prosthesis, aortic valve resuspension (13), or aortic-valve-sparing re-implantation procedure according to David (14). Finally, we classified the extent of aortic vessel surgery according to prosthetic replacement of the ascending aorta only, replacement of the ascending aorta and the aortic arch, both partial and total, and replacement of ascending aorta and entire aortic arch using a conventional or frozen elephant trunk (Table 1) (15).
Full table
Long-term clinical follow-up
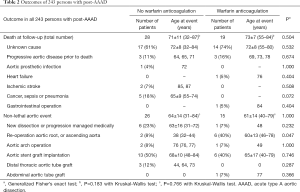
We assessed late postsurgical outcome including death, cause of death, and aortic events in all persons by postsurgical clinical re-evaluation in our out-patient clinics in 200 persons. In addition, we performed follow-up by phone contact in 112 persons. The mean time-interval from surgery to the most recent contact was 44±26 months (range 1–107 months. We analyzed the statistical relationship of long-term outcomes (Table 2) with baseline clinical baseline characteristics (Tables 3 and 4).
Full table
Full table
Full table
Long-term tomographic imaging follow-up
A total of 148 persons underwent postsurgical tomographic imaging of the entire thoracic abdominal aorta in our surgical centers. These comprised 130 persons with contrast-enhanced CT imaging and 18 persons with contrast-enhanced magnetic resonance imaging. The mean time-interval from surgery to the most recent tomographic imaging was 31±24 months (range 1–89 months). In 106 of these 148 persons serial follow-up tomographic imaging was available. We assessed the false lumen status in all 148 persons with postsurgical tomographic imaging procedures, where we used the final postsurgical examinations in those 106 individuals with serial imaging. We classified the false lumen status as patent if flow was present in the absence of thrombus, as partially thrombosed if both flow and thrombus were present, or as completely thrombosed if no flow was present (Table 5) (16). In 107 of those 148 with postoperative tomographic imaging we had access to preoperative tomographic images to analyze the change of the residual false lumen status between preoperative and final postoperative images.

Full table
Finally, we assessed aortic growth rates in 106 persons with serial tomographic imaging from images with at least 6 months of time between first and final follow-up examination (17). We obtained aortic diameters at all standard levels, and we presented the annual aortic growth rate from the aortic segment which exhibited the maximal growth in each patient. We applied current guideline criteria to classify this maximal growth rate as rapid aortic growth with an increase in diameter ≥0.5 cm per year (18,19).
Statistical methods
Unless otherwise specified, we expressed quantitative data as means ± standard deviation and qualitative data as numbers (percentage). For comparison of baseline characteristics, we employed the Kruskal-Wallis test for continuous data and the generalized Fisher’s exact test for nominal and categorical data (Table 1). For time-to-event analysis, we performed univariate Cox proportional hazards regression analysis. We included variables with a P value <0.05 in a multivariable Cox regression model with backward elimination to determine independent predictors of outcome (Tables 3 and 4). We used Kaplan-Meier survival curves to display the occurrence of endpoints over time (Figures 1-3). We used IBM-SPSS software (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY, USA) for all statistical tests.
Results
Frequency and indications for postoperative warfarin anticoagulation
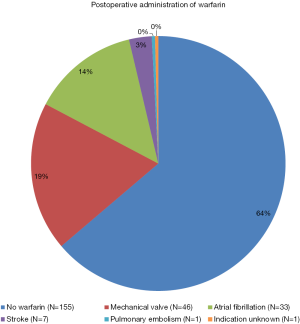
Among 243 postsurgical survivors of AAAD, a total of 88 persons (36%) were put on long-term warfarin before dismissal. In those persons with warfarin anticoagulation, the indication for warfarin was a mechanical aortic prosthesis in 46 (52%), atrial fibrillation in 33 (38%), stroke in 7 (8%), and pulmonary embolism in one (1.1%). Of the 88 persons with warfarin anticoagulation, three were already on anticoagulation prior to the onset of AAAD. In these three persons, the primary indication for warfarin anticoagulation remained unclear in a 74 years old obese hypertensive male (Figure 4). Chronic arterial hypertension, diabetes (both P=0.038), and a mechanical aortic prosthesis (P<0.001) were more prevalent with warfarin anticoagulation. All other characteristics were comparable irrespective of warfarin anticoagulation (Table 1).
Long-term clinical outcome according to warfarin anticoagulation
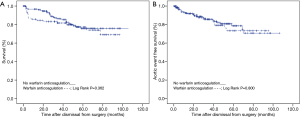
With 28 (18%) versus 19 (22%) late death occurred with similar frequency (P=0.504), and at similar age (P=0.183) irrespective of warfarin anticoagulation. With 26 (17%) versus 15 (17%) non-lethal aortic events also occurred with a similar frequency (P=1.000) and at a similar age (P=0.766) irrespective of warfarin anticoagulation. The causes of death and the types of aortic events were similar in patients irrespective of warfarin anticoagulation (P=0.047; Table 2). Kaplan-Mayer curve analysis showed that over-all survival (P=0.362), and over-all aortic event free survival were similar after surgical repair of AAAD irrespective of warfarin anticoagulation (P=0.800; Figure 1). Moreover, the only two major strokes during follow-up both occurred in patients without warfarin anticoagulation.
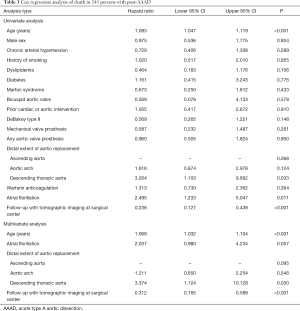
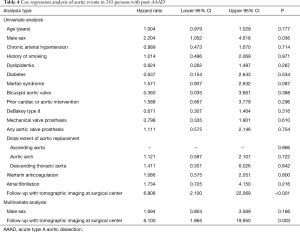
Clinical predictors of long-term outcome
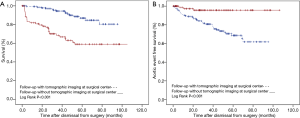
Univariate Cox regression analysis did not identify an association of warfarin anticoagulation with late death (P=0.364; Table 3) or with late aortic events (P=0.800; Table 4). Instead, univariate Cox regression analysis of late death established an association with increased age at operation (P<0.001), operation extending into the descending aorta (P=0.033), atrial fibrillation (P=0.011), and follow-up without tomographic imaging performed at a surgical center (P<0.001). Multivariate Cox regression analysis corroborated higher age (P<0.001), operation extending into the descending aorta (P=0.030), and follow-up without tomographic imaging at a surgical center (P<0.001) as independent predictors of late death (Table 3).
In addition, univariate Cox regression analysis of late aortic events identified an association with male sex (P=0.036), and with follow-up without tomographic imaging at a surgical center (P<0.001). Multivariate Cox regression analysis exclusively corroborated follow-up without tomographic imaging at a surgical center as an independent predictor of late aortic events (P=0.003; Table 4). Kaplan-Meyer analysis documented the association of follow-up without tomographic imaging at a surgical center with both late death and late aortic events (both P<0.001; Figure 2).
Aortic features on follow-up tomographic imaging
There were 148 survivors of AAAD with postoperative tomographic imaging, in whom we assessed the postoperative false lumen status. The postoperative status of the false lumen was similar irrespective of warfarin anticoagulation (Figure 5A). The frequency of persistence of an overt false lumen, and the frequency of the change from preoperative overt false lumen to both partial thrombosis, and complete thrombosis were also similar irrespective of warfarin anticoagulation (Figure 5B). Moreover, the postoperative false lumen status was unrelated to both late death and late aortic events (data not shown).
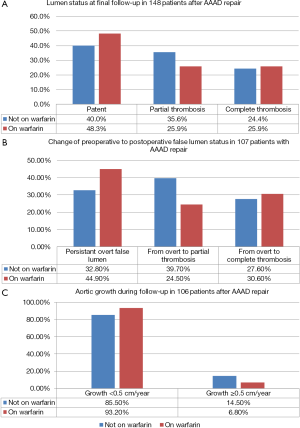
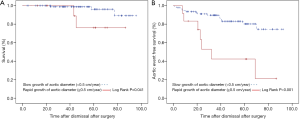
There were 106 survivors of AAAD with serial postoperative tomographic imaging. In these we identified 12 individuals (11%) with rapid aortic growth, defined as an increase of diameters ≥0.5 cm per year. Univariate Cox regression analysis showed a marginal association of rapid aortic growth with late mortality (hazard ratio 5.389; 95% CI, 0.884–32.838; P=0.068) and a significant association with late aortic events (hazard ratio 4.412; 95% CI, 1.788–10.884; P=0.001). Kaplan-Meyer analysis confirmed an association of rapid aortic growth with both late death (P=0.041) and with late aortic events (P<0.001; Figure 3). Rapid aortic growth related to higher age (P=0.075), and to the status of the false lumen (P=0.037), where partial thrombosis of the false lumen was significantly more prevalent in persons with rapid growth (Table 5). Warfarin anticoagulation was unrelated to rapid aortic growth (Figure 5C; Table 5).
Discussion
The study of warfarin anticoagulation in AAAD survivors (WATAS) yielded important results. First, more than one third of AAAD survivors (36%) were put on long-term warfarin anticoagulation postoperatively, where more than half of all individuals required anticoagulation for a mechanical aortic valve prosthesis (52%). Second, patients on long-term warfarin anticoagulation did not carry an increased risk for late death or for late aortic events. Third, absence of follow-up tomographic imaging yielded both significantly higher long-term mortality and significantly lower rates of documented aortic events. Fourth, WATAS identified an association of rapid aortic growth with late death and with late aortic events. Warfarin anticoagulation, however was unrelated to both rapid aortic growth and false lumen status. Finally, an increased age at AAAD and operation extending into the descending aorta emerged as independent clinical predictors of late death.
Warfarin anticoagulation is frequent and administered for vital indications
At the time of postsurgical discharge, 36% of post-AAAD were on warfarin anticoagulation with similar frequency in all age groups. Similarly, one study reported 54% of post-AAAD were on aspirin, clopidogrel or warfarin (8). WATAS showed that mechanical valve prostheses were the leading reason for warfarin anticoagulation, which highlights that aortic valve replacement remains a common technique even in the era of reconstructive aortic valve surgery (20). Atrial fibrillation was the second leading reason for anticoagulation, which is in accordance with a previously published study revealing atrial fibrillation in 17.4% of post-AAAD (21). Ischemic stroke in known to occur in about 10% of AAAD (12), and in WATAS stroke was the third most frequent indication for warfarin anticoagulation. Therefore, WATAS documents that anticoagulation is frequent in AAAD survivors and that anticoagulation is required for vital indications.
Warfarin anticoagulation is unrelated to adverse late outcome
WATAS found that the over-all postsurgical survival of AAAD was 98.3% at 1 year, and 76.4% at 5 years, which was similar to 90% at 1 year, and 72% to 77% at 5 years as documented in a current review (2). Similarly, WATAS revealed a 98.7% and 91.8% freedom from aortic events at 1- and 5-year, respectively. With a range between 95% (22) and 96.9% (23) at 1 year and between 74.7% (23) and 90% (22) at 5 years current studies reported similar rates of freedom from aortic reoperation. The spectrum of re-operations was also similar in WATAS and in other series (24). Therefore, WATAS yielded comparable long-term outcomes as in other current series of AAAD survivors, and it showed that mortality and aortic events were not increased with long-term anticoagulation. Moreover, major strokes occurred only in two patients who were not on warfarin anticoagulation.
Absence of follow-up tomographic imaging predicts late adverse outcome
WATAS showed that 39% of individuals did not undergo tomographic follow-up imaging at tertiary centers. In other tertiary centers more than 68% of surgically treated individuals may not undergo tomographic imaging (25). However, WATAS showed that individuals without tomographic imaging had both significantly higher long-term mortality and significantly lower rates of documented aortic events. It is likely that those individuals without follow-up aortic imaging may have had treatable aortic complications that remained undetected until lethal aortic complications occurred. However, with the absence of both aortic imaging and autopsies the reason of death remained unknown in 25 of a total of 32 late deaths in these patients (78%). Interestingly, those individuals with documented causes of death despite absence of tomographic aortic imaging all died for non-aortic reasons. Therefore, WATAS showed that follow-up tomographic imaging in expert centers is mandatory for long-term survival after surgically repaired AAAD (12,26).
Tomographic imaging findings relate to outcome but not to warfarin anticoagulation
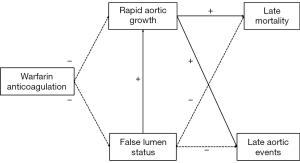
Several studies assessed statistical associations of rapid aortic growth and false lumen status with late death and with late aortic events in postoperative AAAD (12,18,27,28). Figure 6 summarizes the presence or absence of statistical associations among these variables as identified in WATAS. Accordingly, rapid aortic growth is the only predictor of late outcome, whereas false lumen status is related to rapid aortic growth, but not to clinical outcome. Most importantly, warfarin anticoagulation is unrelated to rapid aortic growth, false lumen status and clinical outcomes (Figure 6).
Other clinical predictors of late outcome
Besides warfarin anticoagulation WATAS tested classical clinical variables as candidates for the prediction of late outcome in AAAD survivors. Among a set of 16 variables WATAS identified increased age as an independent predictor of late mortality, which was a well-documented independent predictor of late death (29,30). In addition, WATAS found that extensive distal aortic operations including conventional or frozen elephant trunk operations related to long-term mortality. Such extensive approaches are designed to promote false lumen thrombosis to effectively prevent future aortic events. However, extensive aortic pathology, extensive surgical trauma with prolonged cardiac ischemia, and long duration of cerebral perfusion may combine with individual surgical risk factors to increase early- and long-term mortality in some individuals (15). Other predictors of long-term mortality may be female sex, and a history of atherosclerosis (28), but these factors did not reach statistical significance in WATAS. Interestingly, WATAS identified a univariate association of male sex with late aortic events, where another study confirmed such univariate association of male sex with late aortic reoperation after AAAD repair (31). Finally, WATAS identified a marginal association of Marfan syndrome with late aortic events, but other studies established Marfan syndrome as an independent predictor of late aortic events (22,32). In sum, besides novel predictors, WATAS corroborated classical predictors of late death and late aortic events in AAAD.
Limitations
WATAS is a retrospective observational cohort study with some limits that require elucidation. First, 169 persons had to be excluded from this study (41%), and tomographic imaging data was unavailable in 39% of study participants. Therefore, sampling bias may limit generalization of results. However, our discussion of findings in WATAS documents that cohort characteristics in WATAS corresponded well with other major studies of the postoperative AAAD. Therefore, potential bias did not seem to lead to deviation from cohort characteristics in other studies of the same disease. Second, WATAS data stem from only two tertiary care centers with limited patient numbers, and more data appear mandatory to underpin study results. Further, WATAS identified an aortic cause of death in 13% of late deaths, which was in line with 12% (33) and 24% (32) reported in the literature. However, the cause of death remained unknown in a large number of fatalities. In Germany, there is a dramatic decline of autopsy rates, and therefore only in a minority of deaths an autopsy was performed to elucidate a definitive cause of death (34).
Conclusions
Warfarin anticoagulation is frequent in postsurgical AAAD and it is administered for vital indications. Warfarin anticoagulation does not relate to late mortality or to late aortic events. Rapid aortic growth predicts late mortality and late aortic events, but warfarin anticoagulation is not associated with aortic growth. Follow-up tomographic imaging is mandatory for long-term survival after surgical repair of AAAD.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by regional ethics committee of Hamburg and Dresden (No. EK 317082014), and informed consent was taken from all the patients.
References
- Conzelmann LO, Weigang E, Mehlhorn U, et al. Mortality in patients with acute aortic dissection type A: analysis of pre- and intraoperative risk factors from the German Registry for Acute Aortic Dissection Type A (GERAADA). Eur J Cardiothorac Surg 2016;49:e44-52. [Crossref] [PubMed]
- Krüger T, Conzelmann LO, Bonser RS, et al. Acute aortic dissection type A. Br J Surg 2012;99:1331-44. [Crossref] [PubMed]
- Hirst AE Jr, Johns VJ Jr, Kime SW Jr. Dissecting aneurysm of the aorta: a review of 505 cases. Medicine (Baltimore) 1958;37:217-79. [Crossref] [PubMed]
- Lentini S, Perrotta S. Aortic dissection with concomitant acute myocardial infarction: From diagnosis to management. J Emerg Trauma Shock 2011;4:273-8. [Crossref] [PubMed]
- Cannesson M, Burckard E, Lefevre M, et al. Predictors of in-hospital mortality in the surgical management of acute type A aortic dissections: impact of anticoagulant therapies. Ann Fr Anesth Reanim 2004;23:568-74. [Crossref] [PubMed]
- Lachat M, Criado FJ, Veith FJ. The case for anticoagulation in patients with acute type B aortic dissection. J Endovasc Ther 2008;15:52-3. [Crossref] [PubMed]
- Kantelhardt SR, Pasnoori V, Varma J, et al. Recurrent aortic dissection in Marfan's syndrome: possible effects of anticoagulation. Cardiol Rev 2003;11:240-3. [Crossref] [PubMed]
- Song SW, Yoo KJ, Kim DK, et al. Effects of early anticoagulation on the degree of thrombosis after repair of acute DeBakey type I aortic dissection. Ann Thorac Surg 2011;92:1367-74; discussion 1374-5. [Crossref] [PubMed]
- Shiraishi Y, Kohno T, Egashira T, et al. Thrombus in acute aortic dissection with atrial fibrillation: a treatment dilemma. Am J Emerg Med 2015;33:308.e3-4. [Crossref] [PubMed]
- Morimoto S, Izumi T, Sakurai T, et al. Pulmonary embolism and deep vein thrombosis complicating acute aortic dissection during medical treatment. Intern Med 2007;46:477-80. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495-9. [Crossref] [PubMed]
- Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases. Eur Heart J 2014;35:2873-926. [Crossref] [PubMed]
- Immer FF, Aeschimann R, Englberger L, et al. Resuspension of the aortic valve in acute type A dissection: long-term results. J Heart Valve Dis 2008;17:94-7; discussion 97. [PubMed]
- Bernhardt AM, Treede H, Rybczynski M, et al. Comparison of aortic root replacement in patients with Marfan syndrome. Eur J Cardiothorac Surg 2011;40:1052-7. [PubMed]
- Shrestha M, Bachet J, Bavaria J, et al. Current status and recommendations for use of the frozen elephant trunk technique: a position paper by the Vascular Domain of EACTS. Eur J Cardiothorac Surg 2015;47:759-69. [Crossref] [PubMed]
- Tsai TT, Evangelista A, Nienaber CA, et al. Partial thrombosis of the false lumen in patients with acute type B aortic dissection. N Engl J Med 2007;357:349-59. [Crossref] [PubMed]
- Tolenaar JL, van Keulen JW, Trimarchi S, et al. Number of entry tears is associated with aortic growth in type B dissections. Ann Thorac Surg 2013;96:39-42. [Crossref] [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease. Circulation 2010;121:e266-369. [Crossref] [PubMed]
- Boodhwani M, Andelfinger G, Leipsic J, et al. Canadian Cardiovascular Society position statement on the management of thoracic aortic disease. Can J Cardiol 2014;30:577-89. [Crossref] [PubMed]
- Kallenbach K, Oelze T, Salcher R, et al. Evolving strategies for treatment of acute aortic dissection type A. Circulation 2004;110:Ii243-9. [Crossref] [PubMed]
- Chew HC, Lim SH. Aortic dissection presenting with atrial fibrillation. Am J Emerg Med 2006;24:379-80. [Crossref] [PubMed]
- Zierer A, Voeller RK, Hill KE, et al. Aortic enlargement and late reoperation after repair of acute type A aortic dissection. Ann Thorac Surg 2007;84:479-86; discussion 486-7. [Crossref] [PubMed]
- Kirsch M, Soustelle C, Houel R, et al. Risk factor analysis for proximal and distal reoperations after surgery for acute type A aortic dissection. J Thorac Cardiovasc Surg 2002;123:318-25. [Crossref] [PubMed]
- Cho K, Jeong J, Park J, et al. Long-term changes in the distal aorta after aortic arch replacement in acute debakey type I aortic dissection. Korean J Thorac Cardiovasc Surg 2016;49:264-72. [Crossref] [PubMed]
- Leontyev S, Haag F, Davierwala PM, et al. Postoperative changes in the distal residual aorta after surgery for acute type A aortic dissection: Impact of false lumen patency and size of descending aorta. Thorac Cardiovasc Surg 2017;65:90-8. [PubMed]
- Albrecht F, Eckstein F, Matt P. Is close radiographic and clinical control after repair of acute type A aortic dissection really necessary for improved long-term survival? Interact Cardiovasc Thorac Surg 2010;11:620-5. [Crossref] [PubMed]
- Regeer MV, Martina B, Versteegh MI, et al. Prognostic implications of descending thoracic aorta dilation after surgery for aortic dissection. J Cardiovasc Comput Tomogr 2017;11:1-7. [Crossref] [PubMed]
- Li D, Ye L, He Y, et al. False lumen status in patients with acute aortic dissection: A systematic review and meta-analysis. J Am Heart Assoc 2016;5. [Crossref] [PubMed]
- Rylski B, Hoffmann I, Beyersdorf F, et al. Acute aortic dissection type A: age-related management and outcomes reported in the German Registry for Acute Aortic Dissection Type A (GERAADA) of over 2000 patients. Ann Surg 2014;259:598-604. [Crossref] [PubMed]
- Bruno VD, Chivasso P, Guida G, et al. Surgical repair of Stanford type A aortic dissection in elderly patients: a contemporary systematic review and meta-analysis. Ann Cardiothorac Surg 2016;5:257-64. [Crossref] [PubMed]
- Fukui T, Tabata M, Morita S, et al. Gender differences in patients undergoing surgery for acute type A aortic dissection. J Thorac Cardiovasc Surg 2015;150:581-7.e1. [Crossref] [PubMed]
- Fattouch K, Sampognaro R, Navarra E, et al. Long-term results after repair of type A acute aortic dissection according to false lumen patency. Ann Thorac Surg 2009;88:1244-50. [Crossref] [PubMed]
- Halstead JC, Meier M, Etz C, et al. The fate of the distal aorta after repair of acute type A aortic dissection. J Thorac Cardiovasc Surg 2007;133:127-35.e1. [Crossref] [PubMed]
- Petros K, Wittekind C. Autopsy-a procedure of medical history? Med Klin Intensivmed Notfmed 2014;109:115-20. [Crossref] [PubMed]


